Abstract
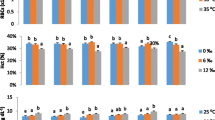
Fertilization and development in salmonids occurs almost exclusively within freshwater environments (< 1 ppt). A less common life history strategy in this group of fishes is the brackish-water resident life history, where entire life cycles occur in brackish water (> 1 ppt). In the present study, we tested the hypothesis that differences in rearing environment (fresh or brackish water) results in significant differences in the ability of lake trout to ionoregulate when faced with a salinity challenge later in life. To test this, genetically similar lake trout were fertilized and raised at either 0 or 5 ppt saltwater. At approximately 240 days post hatch, lake trout from both rearing environments were acutely transferred to 20 ppt salt water or their respective rearing environments as a control. Individuals were sampled at time 0, 1, 7, and 14 days post transfer. Fish raised in 5 ppt transferred to 20 ppt saltwater had significantly higher gill Na+ K+-ATPase activity, gill Na+ K+-ATPase α1b expression, and lower plasma osmolality when compared to freshwater reared lake trout transferred to 20 ppt across various time points. Additionally, the 5 ppt control treatment had greater overall aerobic scope than 0 ppt control fish and those transferred from 0 ppt to 20 ppt. These data imply that populations exhibiting a brackish-water resident life history, as has been observed in Arctic Canada, may have an advantage over freshwater reared conspecifics when foraging in marine influenced environments and colonizing new locations in coastal regions.





Similar content being viewed by others
References
Albert A, Vetemaa M, Saat T (2004) Effects of salinity on the development of Peipsi whitefish Coregonus lavaretus maraenoides Poljakow embryos. Ann Zool Fenn 41:85–88
Alexandrou MA, Swartz BA, Matzke NJ, Oakley TH (2013) Genome duplication and multiple evolutionary origins of complex migratory behavior in Salmonidae. Mol Phylogenet Evol 69:514–523. doi:10.1016/j.ympev.2013.07.026
Altinok I, Grizzle JM (2001) Effects of brackish water on growth, feed conversion and energy absorption efficiency by juvenile euryhaline and freshwater stenohaline fishes. J Fish Biol 59:1142–1152. doi:10.1046/j.1365-2761.2001.00306.x
Atse CB, Audet C, De La Noüe J (2002) Effects of temperature and salinity on the reproductive success of Arctic charr, Salvelinus alpinus (L.): egg composition, milt characteristics and fry survival. Aquac Res 33:299–309. doi:10.1046/j.1355-557x.2002.00674.x
Bystriansky JS, Schulte PM (2011) Changes in gill H+−ATPase and Na+/K+-ATPase expression and activity during freshwater acclimation of Atlantic salmon (Salmo salar). J Exp Biol 214:2435–2442. doi:10.1242/jeb.050633
Bystriansky JS, Richards JG, Schulte PM, Ballantyne JS (2006) Reciprocal expression of gill Na+/K+−ATPase alpha-subunit isoforms α1a and α1b during seawater acclimation of three salmonid fishes that vary in their salinity tolerance. J Exp Biol 209:1848–1858. doi:10.1242/jeb.02188
Bystriansky JS, Frick NT, Richards JG et al (2007) Failure to up-regulate gill Na+,K+-ATPase α-subunit isoform α1b may limit seawater tolerance of land-locked Arctic char (Salvelinus alpinus). Comp Biochem Physiol 148:332–338. doi:10.1016/j.cbpa.2007.05.007
Chabot D, Steffensen JF, Farrell AP (2016) The determination of standard metabolic rate in fishes. J Fish Biol 88:81–121. doi:10.1111/jfb.12845
Chapman BB, Hulthén K, Brodersen J et al (2012a) Partial migration in fishes: causes and consequences. J Fish Biol 81:456–478. doi:10.1111/j.1095-8649.2012.03342.x
Chapman BB, Skov C, Hulthén K et al (2012b) Partial migration in fishes: definitions, methodologies and taxonomic distribution. J Fish Biol 81:479–499. doi:10.1111/j.1095-8649.2012.03349.x
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159. doi:10.1016/0003-2697(87)90021-2
Crespi BJ, Fulton MJ (2004) Molecular systematics of Salmonidae: combined nuclear data yields a robust phylogeny. Mol Phylogenet Evol 31:658–679. doi:10.1016/j.ympev.2003.08.012
Cruz MJ, Sourial MM, Treberg JR et al (2013) Cutaneous nitrogen excretion in the African clawed frog Xenopus laevis: effects of high environmental ammonia (HEA). Aquat Toxicol 136:1–12. doi:10.1016/j.aquatox.2013.03.002
Dodson JJ, Laroche J, Lecomte F (2009) Contrasting evolutionary pathways of anadromy in euteleostean fishes. Am Fish Soc Symp 69:63–77
Engstedt O, Stenroth P, Larsson P et al (2010) Assessment of natal origin of pike (Esox lucius) in the Baltic Sea using Sr:ca in otoliths. Environ Biol Fish 89:547–555. doi:10.1007/s10641-010-9686-x
Evans DO (2007) Effects of hypoxia on scope-for-activity and power capacity of lake trout (Salvelinus namaycush). Can J Fish Aquat Sci 64:345–361. doi:10.1139/f07-007
Finstad AG, Hein CL (2012) Migrate or stay: terrestrial primary productivity and climate drive anadromy in Arctic char. Glob Chang Biol 18:2487–2497. doi:10.1111/j.1365-2486.2012.02717.x
Gibbs A, Somero GN (1989) Pressure adaptation of Na+/K+-ATPase in gills of marine teleosts. J Exp Biol 143:475–492
Gross MR (1987) Evolution of diadromy in fishes. Am Fish Soc Symp 1:14–25
Helle JH, Williamson RS, Bailey JE (1964) Intertidal ecology and life history of pink Salmon at Olsen Creek, Prince Williams sound, Alaska United States. Fish Wildl Serv Spec Sci Rep 483:1–26
Hendry AP, Bohlin T, Jonsson B, Berg OK (2004) To sea or not to sea? Anadromy versus non- Andromy in salmonids. In: Stearns SC (ed) Hendry AP. Evolution Illuminated, Salmon and Their Relatives, pp 92–126
Herrmann JP, Enders EC (2000) Effect of body size on the standard metabolism of horse mackerel. J Fish Biol 57:746–760. doi:10.1111/j.1095-8649.2000.tb00272.x
Himberg K-JM, Lehtonen H (1995) Systematics and nomenclature of coregonid fishes, particularly in Northwest Europe. Arch fur Hybrobiologie Spec Issue Adv Limnol 46:36–47
Hiroi J, McCormick S (2007) Variation in salinity tolerance, gill Na+/K+-ATPase, Na+/K+/2Cl- cotransporter and mitochondria-rich cell distribution in three salmonids Salvelinus namaycush, Salvelinus fontinalis and Salmo salar. J Exp Biol 210:1015–1024. doi:10.1242/jeb.002030
Jonsson B, Johnsson N (2001) Polymorphism and speciation in Arctic charr. J Fish Biol 58:605–638. doi:10.1006/jfbi.2000.1515
Jonsson B, Jonsson N (1993) Partial migration: niche shift versus sexual maturation in fishes. Rev Fish Biol Fish 3:348–365
Kissinger BC, Gantner N, Anderson WG et al (2016) Brackish-water residency and semi-anadromy in Arctic lake trout (Salvelinus namaycush) inferred from otolith microchemistry. J Great Lakes Res 42:267275. doi:10.1016/j.jglr.2015.05.016
Marshall WS, Bryson SE (1998) Transport mechanisms of seawater teleost chloride cells: an inclusive model of a multifunctional cell. Comp Biochem Physiol 119:97–106. doi:10.1016/S1095-6433(97)00402-9
McCormick SD (1993) Methods for nonlethal gill biopsy and measurement of Na+, K+−ATPase activity. Can J Fish Aquat Sci 50:656–658
McCormick SD (1994) Ontogeny and evolution of salinity tolerance in anadromous salmonids: hormones and heterochrony. Estuaries 17:26–33. doi:10.1007/BF02694900
McCormick SD (1995) Hormonal control of gill Na+, K+-ATPase and chloride cell function. In: wood CM, Shuttleworth TJ (eds) cellular and molecular approaches to fish ionic regulation: fish physiology, 14th edn. Academic Press, pp 285–315
McCormick SD (1996) Effects of growth hormone and insulin-like growth factor I on salinity tolerance and gill Na+, K+−ATPase in Atlantic salmon (Salmo salar): interaction with cortisol. Gen Comp Endocrinol 101:3–11. doi:10.1006/gcen.1996.0002
McCormick SD (2001) Endocrine control of osmoregulation in teleost fish. Am Zool 41:781–794
McCormick SD (2009) Evolution of the hormonal control of animal performance: insights from the seaward migration of salmon. Integr Comp Biol 49:408–422. doi:10.1093/icb/icp044
McCormick SD, Saunders RL (1987) Preparatory physiological adaptations for marine life of salmonids: osmoregulation, growth, and metabolism. Am Fish Soc Symp 1:211–229
McCormick SD, Naiman RJ, Montgomery ET (1985) Physiological smolt characteristics of anadromous and non-anadromous brook trout (Salvelinus fontinalis) and Atlantic salmon (Salmo salar). Can J Fish Aquat Sci 42:529–538. doi:10.1139/f85-070
Morgan JD, Iwama GK (1991) Effects of salinity on growth, metabolism, and ion regulation in juvenile rainbow and steelhead trout (Oncorhynchus mykiss) and fall Chinook Salmon (Oncorhynchus tshawytcha). Can J Fish Aquat Sci 48:2083–2094
Morgan JD, Iwama GK (1998) Salinity effects on oxygen consumption, gill Na+, K+-ATPase and ion regulation in juvenile coho salmon. J Fish Biol 53:1110–1119. doi:10.1006/jfbi.1998.0780
Norin T, Malte H (2011) Repeatability of standard metabolic rate, active metabolic rate and aerobic scope in young brown trout during a period of moderate food availability. J Exp Biol 214:1668–1675. doi:10.1242/jeb.054205
Papakostas S, Vasemägi A, Vähä J-P et al (2012) A proteomics approach reveals divergent molecular responses to salinity in populations of European whitefish (Coregonus lavaretus). Mol Ecol 21:3516–3530. doi:10.1111/j.1365-294X.2012.05553.x
Perrott MN, Grierson CE, Hazon N, Balment RJ (1992) Drinking behaviour in sea water and fresh water teleosts, the role of the renin-angiotensin system. Fish Physiol Biochem 10:161–168. doi:10.1007/BF00004527
Scott WB, Crossman EJ (1973) Freshwater fishes of Canada. The Bryant Press Limited, Ottawa
Stearns SC (1995) The evolution of life histories. Oxford University Press, New York
Svendsen JC, Steffensen JF, Aarestrup K et al (2012) Excess posthypoxic oxygen consumption in rainbow trout (Oncorhynchus mykiss): recovery in normoxia and hypoxia. Can J Zool 90:1–11. doi:10.1139/z11-095
Swanson HK, Kidd KA, Babaluk JA et al (2010) Anadromy in Arctic populations of lake trout (Salvelinus namaycush): otolith microchemistry, stable isotopes, and comparisons with Arctic char (Salvelinus alpinus). Can J Fish Aquat Sci 67:842–853. doi:10.1139/F10-022
Wertheimer AC (1984) Maturation success of pink salmon (Oncorhynchus gorbuscha) and coho salmon (O. kisutch) held under three salinity regimes. Aquaculture 43:195–212. doi:10.1016/0044-8486(84)90022-X
Wilson CC, Hebert PD (1998) Phylogeography and postglacial dispersal of lake trout (Salvelinus namaycush) in North America. Can J Fish Aquat Sci 55:1010–1024. doi:10.1139/f97-286
Acknowledgements
This project was made possible through funding and collaboration from multiple sources. Funding was provided by NSERC Discovery grant (#311909) to WGA, JRT was funded by an NSERC Discovery Grant (#418503) and the Canada Research Chairs Program (#223744). Assistance in fish rearing was supported by the University of Manitoba Animal holding staff and extensive support and guidance was given by Terry Smith. Additional assistance, with fish husbandry and sampling was provided by Alex Borecky, Ben Carriere, Alex Hare, Biobelemoye Irabor, and Julia Wiens. Assistance with statistics was provided by Dr. David Deslauriers and editing by Amy Flasko. Gamete collection was approved by Manitoba Conservation and Water Stewardship fisheries science and fish culture permit number 31-13 and all subsequent experiments were approved under the Animal Use Protocol F13-029 by the University of Manitoba protocol management review committee following guidelines established by the Canadian Council for Animal Care. We are grateful to provincial fisheries biologists, Laureen Janusz and Jeff Long, for their assistance in the capture and harvesting of gametes from wild-caught lake trout.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kissinger, B.C., Bystriansky, J., Czehryn, N. et al. Environment-phenotype interactions: Influences of brackish-water rearing on lake trout (Salvelinus namaycush) physiology. Environ Biol Fish 100, 797–814 (2017). https://doi.org/10.1007/s10641-017-0607-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-017-0607-0