Abstract
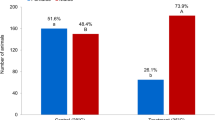
Sex determination in fishes is often enigmatic, a situation that is often made even more complex by the fact that the process of sexual differentiation in many species may be influenced by environmental conditions. This situation is typified in zebrafish, a popular model organism. Despite the vast array of information available for the species, the genetic controls of sex are unknown. Further, environmental parameters, such as rearing densities, seem to exert an influence on the sex ratios of captive stocks. In an effort to dissect the genetic and environmental controls underlying the expression of sex in this species, we manipulated growth of pure-bred and out-crossed zebrafish by varying their food supply during development. Faster-growing zebrafish were more likely to be female than siblings that were fed less, and out-crossed broods had higher proportions of females than broods from pure-bred crosses. The dependence of sex ratio on feeding rate is readily understood in terms of adaptive sex allocation: zebrafish life history seems to confer the greater pay-off for large size on females. A similar male/female difference in the pay-off for hybrid vigor could similarly account for the female bias of out-crossed broods—and it could be a manifestation of Haldane’s rule.

Similar content being viewed by others
References
Anderson L, Holbech H, Gessbo A, Norgrenn L, Petersen G (2003) Effects of exposure to 17a-ethinylestradiol during early development on sexual differentiation and induction of vitellogenin in zebrafish (Danio rerio). Comp Physiol Biochem Part C: Toxicol Pharmacol 134:365–374
Blazquez M, Carrillo M, Zanuy S, Piferrer F (1999) Sex ratios in offspring of sex-reversed sea bass and the relationship between growth and phenotypic sex differentiation. J Fish Biol 55:916–930
Charnov EL, Bull JJ (1977) When is sex environmentally determined? Nature 266:828–830
Conover DO, Heins SW (1987) Adaptive variation in environmental and genetic sex determination in a fish. Nature 326:496–498
Conover DO, Kynard BE (1981) Environmental sex determination: interaction of temperature and genotype in a fish. Science 213:577–579
Fishman MC (2001) Genomics. Zebrafish – the canonical vertebrate. Science 294:1290–1291
Francis RC (1984) Experimental effects on agonistic behavior in the paradise fish, Macropodus opercularus. Behaviour 85:292–313
Francis R, Barlow G (1993) Social control of primary sex differentiation in the Midas cichlid. Proc Natl Acad Sci USA 90:10673–10675
Fujioka Y (2001) Thermolabile sex determination in honoroko. J Fish Biol 59:851–861
Godwin J, Luckenbach JA, Borski RJ (2003) Ecology meets endocrinology: environmental sex determination in fishes. Evol Dev 5:40–49
Haldane JBS (1922) Sex-ratio and unisexual sterility in hybrid animals. J Genet 12:101–109
Holloway AC, Leatherland JF (1998) Neuroendocrine regulation of growth hormone secretion in teleost fishes with emphasis on the involvement of gonadal sex steroids. Rev Fish Biol Fisher 8:409–429
Kitano T, Takamune K, Kobayashi T, Nagahama Y, Abe SI (1999) Suppression of P450 aromatase gene expression in sex-reversed males produced by rearing genetically female larvae at high water temperature during a period of sex differentiation in the Japanese flounder Paralichthys olivaceus. J Mol Endocrinol 23:167–176
Kraak SBM, de Looze EMA (1993) A new hypothesis on the evolution of sex determination in vertebrates: big females ZW, big males XY. Neth J Zoo 43:260–273
Krueger WH, Oliveira K (1999) Evidence for environmental sex determination in the American eel, Anguilla rostrata. Env Biol Fish 55:381–389
Lorenzen K, Enberg K (2002) Density-dependent growth as a key mechanism in the regulation of fish populations: evidence from among-population comparisons. Proc Roy Soc London B 269:49–54
Menon AGK (1999) Check list – fresh water fishes of India. Occasional paper no. 175, 366, pp 234–259
Mitton JB (1993) Enzyme heterozygosity, metabolism, and developmental stability. Genetica 89:47–65
Nechiporuk A, Finney JA, Keating MT, Johnson SL (1999) Assessment of polymorphism in zebrafish mapping strains. Genome Res 9:1231–1238
Nusslein-Volhard C, Dahm R (2002) Zebrafish: a practical approach. Oxford University Press, Oxford, UK
Orr AH (2005) The genetic basis of reproductive isolation: insights from Drosophila. Proc Natl Acad Sci USA 102:6522–6526
Pelegri F, Schulte-Merker S (1999) A gynogenesis-based screen for maternal-effect genes in the zebrafish, Danio rerio. Meth Cell Biol 60:1–20
Piferrer FS, Zanuy S, Carrillo M, Solar II, Devlin RH, Donaldson EM (1994) Brief treatment with an aromatase inhibitor during sex differentiation causes chromosomally female salmon to develop as normal functional males. J Exp Zool 270:255–262
Piferrer F (2001) Endocrine sex control strategies for the feminization of teleost fish. Aquaculture 197:229–281
Pritchard VL, Lawrence J, Butlin RK, Krause J (2001) Shoal choice in zebrafish, Danio rerio: influence of shoal size and activity. Anim Behav 62:1085–1088
Pyron M (2003) Female preferences and male–male interactions in zebrafish (Danio rerio). Can J Zool 81:122–125
Rahman AKA (1989) Freshwater fishes of Bangladesh. Zool. Soc. Bangladesh, Dept. Zool., Univ. of Dhaka
Reimchen TE, Nosil P (2001) Lateral plate asymmetry, diet and parasitism in threespine stickleback. J Evol Biol 14:632–645
Spence R, Smith C (2004) Male territoriality mediates density and sex ratio effects on oviposition in the zebrafish, Danio rerio. Anim Behav 69:1317–1323
Talwar PK, Jhingran AG (1991) Inland fishes of India and adjacent countries, vol 1. A.A. Balkema, Rotterdam
Tooby J (1982) Pathogens, polymorphism and the evolution of sex. J Theor Biol 97:557–576
Traut W, Winking H (2001) Meotic chromosomes and stages of sex chromosome evolution in fish: zebrafish, platyfish, and guppy. Chrom Res 9:659–672
Uchida D, Yamashita M, Kitano T, Iguchi T (2002) Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J Exp Biol 205:711–718
Uchida D, Yamashita M, Kitano T, Iguchi T (2004) An aromatase inhibitor or high water temperature induces oocyte apoptosis and depletion of P450 aromatase activity in the gonads of genetic female zebrafish during sex-reversal. Comp Biochem Physiol A 137:11–20
Acknowledgments
We thank Barry Paw for his support during these experiments. We are also grateful to Karel Liem and Hans Hoffman for helpful discussions during preliminary stages of the project, and Tracey Spoon, Carl Smith, and Les Kaufman for reviewing various versions of the paper. Finally, we also thank the three referees for their helpful criticisms of the manuscript. All experiments comply with current laws of the USA and were conducted in adherence to guidelines set forth by the institutional animal care and use committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lawrence, C., Ebersole, J.P. & Kesseli, R.V. Rapid growth and out-crossing promote female development in zebrafish (Danio rerio). Environ Biol Fish 81, 239–246 (2008). https://doi.org/10.1007/s10641-007-9195-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-007-9195-8