Abstract
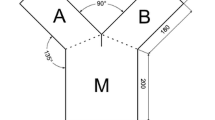
We studied the effect of cyanobacteria on foraging and refuge use in small fish. We measured pike larval feeding in the presence of cyanobacteria by counting leftover prey. Our results showed that feeding by pike larvae on zooplankton prey decreased significantly in the presence of non-toxic cyanobacteria. The behaviour can be due to lowered vision caused by turbidity or clogging of the gills. Further, we tested whether the three-spined stickleback use toxic cyanobacteria as a refuge against predators in a choice experiment. The choice experiment was performed in a Y-maze fluviarum, where the fish could select between two different environments. Our results support the refuge use hypothesis because the three-spined stickleback clearly preferred toxic cyanobacteria to the chemical predator signal. To conclude, cyanobacteria decrease feeding rates in fish larvae, but may function as important refuge for e.g. sticklebacks, during predation pressure in pelagic algal blooms.
Similar content being viewed by others
References
Baganz D, Staaks G, Pflugmacher S, Steinberg CEW (2004) Comparative study of microcystin-LR-induced behavioural changes of two fish species, Danio rerio and Leucaspius delineatus. Environ Toxicol 19:564–570
Baganz D, Staaks G, Steinberg C (1998) Impact of the cyanobacteria toxin, microcystin-LR on behaviour of zebrafish, Danio rerio. Water Res 32:948–952
Brown SB, Evans RE, Thompson BE, Hara TJ (1982) Chemoreception and aquatic pollutants. In: Hara TJ (ed) Chemoreception in fishes. Developments in aquaculture and fisheries science. Vol 8. Elsevier Science Publishers, Amsterdam, pp 363–293
Casselman JM (1996) Age, growth and environmental requirements of pike. In: Craig JF (ed) Pike: biology and exploitation. Chapman, Hall, London, pp 70–101
Craig JF, Babaluk JA (1989) Relationship of condition of walleye (Stizostedion vitreum) and northern pike (Esox lucius) to water clarity, with special reference to Dauphin Lake, Manitoba. Can J Fish Aquat Sci 46:1581–1586
Csányi V (1985) Ethological analysis of predator avoidance by the paradise fish (Macropodus opercularis L). I. Recognition and learning of predators. Behaviour 92:227–240
De Robertis A, Ryer CH, Veloza A, Brodeur RD (2003) Differential effects of turbidity on prey consumption of piscivorous and planktivorous fish. Can J Fish Aquat Sci 60:1517–1526
Dugatkin LA, Godin J-GJ (1992) Prey approaching predators: a cost-benefit perspective. Ann Zool Fenn 29:233–252
Elmgren R (1989) Man’s impact on the ecosystem of the Baltic Sea: energy flows today and at the turn of the century. Ambio 18:326–332
Engström J, Viherluoto M, Viitasalo M (2001) Effects of toxic and non-toxic cyanobacteria on grazing, zooplanktivory and survival of the mysid shrimp Mysis mixta. J Exp Mar Biol Ecol 257:269–280
Engström-Öst J, Koski M, Schmidt K, Viitasalo M, Jónasdóttir SH, Kokkonen M, Repka S, Sivonen K (2002) Effects of toxic cyanobacteria on a plankton assemblage: community development during decay of Nodularia spumigena. Mar Ecol Prog Ser 232:1–14
Finni T, Kononen K, Olsonen R, Wallström K (2001) The history of cyanobacterial blooms in the Baltic Sea. Ambio 30:172–178
Fujii K, Sivonen K, Adachi K, Noguchi K, Shimizo Y, Sano H, Hirayama K, Suzuki M, Harada K (1997a) Comparative study of toxic and non-toxic cyanobacterial products: a novel glycoside, suomilide, from non-toxic Nodularia spumigena HKVV. Tetrahedron Lett 38:5529–5532
Fujii K, Sivonen K, Adachi K, Noguchi K, Shimizo Y, Sano H, Hirayama K, Suzuki M, Harada K (1997b) Comparative study of toxic and non-toxic cyanobacterial products: novel peptides from toxic Nodularia spumigena AV1. Tetrahedron Lett 38:5525–5528
Gregory RS (1993) Effect of turbidity on the predator avoidance behaviour of juvenile chinook salmon (Oncohynchus tshawytscha). Can J Fish Aquat Sci 50:241–246
Gregory RS, Northcote TG (1993) Surface, planktonic, and benthic foraging by juvenile chinook salmon (Oncorhynchus tshawytscha) in turbid laboratory conditions. Can J Fish Aquat Sci 50:233–240
Hara TJ (1993) Role of olfaction in fish behaviour. In: Pitcher TJ (ed) Behaviour of teleost fish. Chapman, Hall, London, pp 171–199
Hirvonen H, Ranta E, Piironen J, Laurila A, Peuhkuri N (2000) Behavioural responses of naive Arctic charr young to chemical cues from salmonid and non-salmonid fish. Oikos 88:191–199
Hoppe H-G (1981) Blue-green algae agglomeration in surface water: a microbiotope of high bacterial activity. Kiel Meeresforsch (Sonderh.) 5:291–303
Hughes EO, Gorham PR, Zehnder A (1958) Toxicity of a unialgal culture of Microcystis aeruginosa. Can J Microbiol 4:225–236
Kahru M, Horstmann U, Rud O (1994) Satellite detection of increased cyanobacteria blooms in the Baltic Sea: natural fluctuation or ecosystem change? Ambio 23:469–472
Kamjunke N, Mehner T (2001) Coupling the microbial food web with fish: are bacteria attached to cyanobacteria an important food source for underyearling roach? Freshw Biol 46:633–639
Kamjunke N, Schmidt K, Pflugmacher S, Mehner T (2002) Consumption of cyanobacteria by roach (Rutilus rutilus): useful or harmful to the fish? Freshw Biol 47:243–250
Kankaanpää HT, Sipiä VO, Kuparinen JS, Ott JL, Carmichael WW (2001) Nodularin analyses and toxicity of Nodularia spumigena (Nostocales, Cyanobacteria) waterbloom in the western Gulf of Finland, Baltic Sea, in August 1999. Phycologia 40:268–274
Karjalainen M, Reinikainen M, Spoof L, Meriluoto J, Sivonen K, Viitasalo M (2005) Trophic transfer of cyanobacterial toxins from zooplankton to planktivores: consequences for pike larvae and mysid shrimps. Environ Toxicol 20:354–362
Keshavanath P, Beveridge MCM, Baird DJ, Lawton LA, Nimmo A, Codd GA (1994) The functional grazing response of a phytoplanktivorous fish Oreochromis niloticus to mixtures of toxic and non-toxic strains of the cyanobacterium Microcystis aeruginosa. J Fish Biol 45:123–129
Kononen K (1992) Dynamics of the toxic cyanobacteria blooms in the Baltic Sea. PhD thesis, University of Helsinki, Finland. Finn Mar Res 261:3–36
Kotai J (1972) Instructions for preparation of modified nutrient solution Z8 for algae. Norwegian Inst. Water Res. Oslo B 11/69:1–5
Laamanen M (2002) Genetic and species diversity of planktonic cyanobacteria in the northern Baltic Sea. Ph.D. thesis, University of Helsinki, 51 pp
Lehtimäki J, Sivonen K, Luukkainen R, Niemelä SI (1994) The effects of incubation time, temperature, light, salinity, and phosphorus on growth and hepatotoxin production by Nodularia strains. Arch Hydrobiol 130:269–282
Lehtiniemi M, Engström-Öst J, Viitasalo M (2005) Turbidity decreases anti-predator behaviour in pike larvae (Esox lucius). Environ Biol Fishes 37:1–8
Leinikki J (1995) The diet of three-spined stickleback in the Gulf of Bothnia during its open water phase. Aqua Fenn 25:71–75
Lemmetyinen R, Mankki J (1975) The three-spined stickleback (Gasterosteus aculeatus) in the food chain of the northern Baltic Sea. Merentutkimuslait Julk/Havsforskningsinst Skr 239:155–161
Mackie AM (1982) Identification of the gustatory feeding stimulants. In: Hara TJ (ed) Chemoreception in fishes. Developments in aquaculture and fisheries science. Vol 8, Elsevier Science Publishers, Amsterdam, pp 275–291
Maes J, Taillieu A, Van Damme PA, Cottenie K, Ollevier F (1998) Seasonal patterns in the fish and crustacean community of a turbid temperate estuary (Zeeschelde Estuary, Belgium). Estuar Coast Shelf Sci 47:143–151
Magurran AE, Girling SL (1986) Predator model recognition and response habituation in shoaling minnows. Anim Behav 34:510–518
Peltonen H, Vinni M, Lappalainen A, Pönni J (2004) Spatial feeding patterns of herring (Clupea harengus L), sprat (Sprattus sprattus L), and the three-spined stickleback (Gasterosteus aculeatus L) in the Gulf of Finland, Baltic Sea. ICES J Mar Sci 61:966–971
Peñaloza R, Rojas M, Vila I, Zambrano F (1990) Toxicity of a soluble peptide from Microcystis sp. to zooplankton and fish. Freshw Biol 24:233–240
Perttilä M, Niemistö L, Mäkelä K (1995) Distribution, development and total amounts of nutrients in the Gulf of Finland. Estuar Coast Shelf Sci 41:345–360
Potter IC, Loneragan NR, Lenanton RCJ, Chrystal PJ (1983) Blue-green algae and fish population changes in a eutrophic estaury. Mar Pollut Bull 14:228–233
Roberts RJ (1978) Fish pathology. Bailliere Tindall, London 319 pp
Sandén P, Rahm L (1993) Nutrient trends in the Baltic Sea. Environmetrics 4:75–103
Sandström A (2004) The influence of visual conditions on young percids (Percidae spp.). PhD thesis, Åbo Akademi University. 72 pp
Shapiro J (1990) Current beliefs regarding dominance by blue-greens: the case for the importance of CO2 and pH. Int Ver Theor Angew Limnol Ver 24:38–54
Sivonen K, Jones G (1999) Cyanobacterial toxins. In: Chorus I, Bartram J (eds) Toxic cyanobacteria in water. A guide to their public health consequences, monitoring and management. E & FN Spon, London, pp 41–111
Smith RJF (1997) Avoiding and deterring predators. In: Godin J-GJ (eds) Behavioural ecology of teleost fishes. Oxford University Press, Oxford, pp 163–190
Snickars M, Sandström A, Mattila J (2004) Anti-predator behaviour of 0+ year Perca fluviatilis: effect of vegetation density and turbidity. J Fish Biol 65:1604–1613
Stuart-Smith RD, Richardson AMM, White RWG (2004) Increasing turbidity significantly alters the diet of brown-trout: a multi-year longitudinal study. J Fish Biol 65: 376–388
Tursch B (1982) Chemical protection of a fish by a soft coral. J Chem Ecol 8:1421–1428
Urho L (2002) The importance of larvae and nursery areas for fish production. PhD thesis, University of Helsinki and Finnish Game and Fisheries Research Institute. 118 pp
Utne-Palm AC (2002) Visual feeding of fish in a turbid environment: physical and behavioural aspects. Mar Freshw Behav Physiol 35:111–128
Viherluoto M (2001) Food selection and feeding behaviour of Baltic Sea mysid shrimps. PhD thesis, University of Helsinki. 35 pp
Wootton RJ (1999) Ecology of teleost fishes. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 386
Yamamori K, Nakamura M, Matsui T, Hara TJ (1988) Gustatory responses to tetrodotoxin and saxitoxin in fish: a possible mechanism for avoiding marine toxins. Can J Fish Aquat Sci 45:2182–2186
Zar JH (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River, USA, pp 663
Acknowledgements
Two anonymous referees are thanked for valuable comments on the manuscript. We thank H. Strandberg for the pike larvae and M. Lehtiniemi for discussions. R. Olsonen and M. Öst advised on statistical issues. T. Sjölund made the fluviarum. The cyanobacteria strains (HKVV and AV1) originated from the culture collection of Prof. K. Sivonen, Department of Chemistry and Microbiology, University of Helsinki. H. Kankaanpää provided us with cyanobacteria when we had run out. J. Lindeberg was responsible for animal care in 2004. The experiments comply with the current laws of Finland. Animal welfare was respected during all stages of the study. Permissions (no: 347 and 69/04) were granted by the Animal Care Committee at the University of Helsinki. Funding by the Academy of Finland (project nos. 202437 and 202382) and Maj and Tor Nessling Foundation is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Engström-Öst, J., Karjalainen, M. & Viitasalo, M. Feeding and Refuge Use by Small Fish in the Presence of Cyanobacteria Blooms. Environ Biol Fish 76, 109–117 (2006). https://doi.org/10.1007/s10641-006-9013-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-006-9013-8