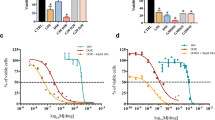
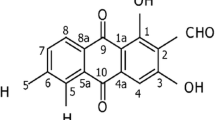
Abstract
Cancer is a disease caused by uncontrolled cell growth that is responsible for several deaths worldwide. Breast cancer is the most common type of cancer among women and is the leading cause of death. Chemotherapy is the most commonly used treatment for cancer; however, it often causes various side effects in patients. In this study, we evaluate the antineoplastic activity of a parent compound based on a combretastatin A4 analogue. We test the compound at 0.01 mg mL− 1, 0.1 mg mL− 1, 1.0 mg mL− 1, 10.0 mg mL− 1, 100.0 mg mL− 1, and 1,000.0 mg mL− 1. To assess molecular antineoplastic activity, we conduct in vitro tests to determine the viability of Ehrlich cells and the blood mononuclear fraction. We also analyze the cytotoxic behavior of the compound in the blood and blood smear. The results show that the molecule has a promising antineoplastic effect and crucial anticarcinogenic action. The toxicity of blood cells does not show statistically significant changes.




Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Siegel RL, Miller KD, Wagle NS, Jemal A (2023) Cancer statistics, 2023. CA: A Cancer. J Clin 73:17–48. https://doi.org/10.3322/caac.21763
Cancer - PAHO/WHO| Pan American Health Organization https://www.paho.org/en/topics/cancer. Accessed 27 Dec 2023
Cancer https://www.who.int/health-topics/cancer. Accessed 27 Dec 2023
Santos MDO, Lima FCDSD, Martins LFL et al (2023) Estimativa De Incidência De Câncer no Brasil, 2023–2025. https://doi.org/10.32635/2176-9745.RBC.2023v69n1.3700. Rev Bras Cancerol 69:
Wendt C, Margolin S (2019) Identifying breast cancer susceptibility genes - a review of the genetic background in familial breast cancer. Acta Oncol 58:135–146. https://doi.org/10.1080/0284186x.2018.1529428
PDQ Adult Treatment Editorial Board (2002) Breast Cancer Treatment (PDQ®): Health Professional Version. PDQ Cancer Information Summaries. National Cancer Institute (US). Bethesda (MD)
Debela DT, Muzazu SG, Heraro KD et al (2021) New approaches and procedures for cancer treatment: current perspectives. SAGE Open Med 9:20503121211034366. https://doi.org/10.1177/20503121211034366
Pantziarka P, Capistrano IR, De Potter A et al (2021) An Open Access Database of Licensed Cancer drugs. Front Pharmacol 12:627574. https://doi.org/10.3389/fphar.2021.627574
Anticancerfund (ed) (2021) https://www.anticancerfund.org/en/cancerdrugs-db. Accessed 27 Dec 2023
Chow LWC, Biganzoli L, Leo AD et al (2017) Toxicity profile differences of adjuvant docetaxel/cyclophosphamide (TC) between Asian and caucasian breast cancer patients. Asia Pac J Clin Oncol 13:372–378. https://doi.org/10.1111/ajco.12682
Kim S, Kang SI, Kim S, Kim JH (2021) Prognostic implications of Chemotherapy-Induced Neutropenia in Stage III Colorectal Cancer. J Surg Res 267:391–396. https://doi.org/10.1016/j.jss.2021.05.002
Tao G, Huang J, Moorthy B et al (2020) Potential role of drug metabolizing enzymes in chemotherapy-induced gastrointestinal toxicity and hepatotoxicity. Expert Opin Drug Metab Toxicol 16:1109–1124. https://doi.org/10.1080/17425255.2020.1815705
Akbarali HI, Muchhala KH, Jessup DK, Cheatham S (2022) Chemotherapy induced gastrointestinal toxicities. Adv Cancer Res 155:131–166. https://doi.org/10.1016/bs.acr.2022.02.007
da Silva EB, Chagas CS, Oliveira MPA et al (2019) Comparative study on myelotoxic and antineoplastic action of Synadenium umbellatum, Vitis vinifera and Resveratrol. J Nat Remedies 80–86. https://doi.org/10.18311/jnr/2019/23383
Safwat MA, Kandil BA, Elblbesy MA et al (2020) Epigallocatechin-3-gallate-loaded Gold nanoparticles: Preparation and evaluation of Anticancer Efficacy in Ehrlich Tumor-Bearing mice. Pharmaceuticals (Basel) 13:254. https://doi.org/10.3390/ph13090254
Brito WAS, Freund E, Nascimento TDH do, et al (2021) The Anticancer efficacy of plasma-oxidized saline (POS) in the Ehrlich Ascites Carcinoma Model in Vitro and in vivo. Biomedicines 9:932. https://doi.org/10.3390/biomedicines9080932
Fernandes PD, Guerra FS, Sales NM et al (2015) Characterization of the inflammatory response during Ehrlich ascitic tumor development. J Pharmacol Toxicol Methods 71:83–89. https://doi.org/10.1016/j.vascn.2014.09.001
Ozaslan M, Karagoz ID, Kilic IH, Guldur ME (2011) Ehrlich ascites carcinoma. Afr J Biotechnol 10:2375–2378. https://doi.org/10.4314/ajb.v10i13
Nik ME, Momtazi-Borojeni AA, Zamani P et al (2019) Targeted-nanoliposomal combretastatin A4 (CA-4) as an efficient antivascular candidate in the metastatic cancer treatment. J Cell Physiol 234:14721–14733. https://doi.org/10.1002/jcp.28230
Paidakula S, Nerella S, Kankala S, Kankala RK (2022) Recent trends in tubulin-binding combretastatin A-4 analogs for Anticancer Drug Development. Curr Med Chem 29:3748–3773. https://doi.org/10.2174/0929867328666211202101641
Chen Z-H, Xu R-M, Zheng G-H et al (2023) Development of Combretastatin A-4 Analogues as potential Anticancer agents with Improved Aqueous solubility. Molecules 28:1717. https://doi.org/10.3390/molecules28041717
de Figueiredo LP, Ibiapino AL, Amaral DN, do et al (2017) Structural characterization and cytotoxicity studies of different forms of a combretastatin A4 analogue. J Mol Struct 1147:226–234. https://doi.org/10.1016/j.molstruc.2017.06.093
do Amaral DN, Cavalcanti BC, Bezerra DP et al (2014) Docking, synthesis and antiproliferative activity of N-Acylhydrazone derivatives designed as Combretastatin A4 analogues. PLoS ONE 9:e85380. https://doi.org/10.1371/journal.pone.0085380
Silva JC, Júnior RG, de O, Silva MG e, et al (2018) LASSBio-1586, an N-acylhydrazone derivative, attenuates nociceptive behavior and the inflammatory response in mice. PLoS ONE 13:e0199009. https://doi.org/10.1371/journal.pone.0199009
Karatoprak GŞ, Küpeli Akkol E, Genç Y et al (2020) Combretastatins: an overview of structure, probable mechanisms of action and potential applications. Molecules 25:2560. https://doi.org/10.3390/molecules25112560
Sherbet GV (2020) Combretastatin analogues in cancer biology: a prospective view. J Cell Biochem 121:2127–2138. https://doi.org/10.1002/jcb.29342
Shahrasbi A, Armin A, Ardebili A et al (2017) Hematologic adverse effects following systemic chemotherapy. 2:1–3. https://doi.org/10.4172/2576-3857.1000110
de Oliveira Gozzo T, Nascimento TG, do, Panobianco MS, de Almeida AM (2011) Ocurrencia de neutropenia en mujeres con cáncer de mama durante El Tratamiento quimioterápico. Acta Paulista De Enfermagem 24:810–814. https://doi.org/10.1590/s0103-21002011000600014
Niraj J, Färkkilä A, D’Andrea AD (2019) The Fanconi Anemia Pathway in Cancer. Annu Rev Cancer Biol 3:457–478. https://doi.org/10.1146/annurev-cancerbio-030617-050422
Gilreath JA, Rodgers GM (2020) How I treat cancer-associated anemia. Blood 136:801–813. https://doi.org/10.1182/blood.2019004017
Abiri B, Vafa M (2020) Iron Deficiency and Anemia in Cancer patients: the role of Iron Treatment in Anemic Cancer patients. Nutr Cancer 72:864–872. https://doi.org/10.1080/01635581.2019.1658794
Wang Y-J, Fletcher R, Yu J, Zhang L (2018) Immunogenic effects of chemotherapy-induced tumor cell death. Genes Dis 5:194–203. https://doi.org/10.1016/j.gendis.2018.05.003
Shrivastava S, Swati Shrivastava, Singh N et al (2016) Comparative study of hematological parameters along with effect of chemotherapy and radiotherapy in different stages of breast cancer. Int J Res Med Sci 5:311–315. https://doi.org/10.18203/2320-6012.ijrms20164569
Coiffier B, Guastalla J-P, Pujade-Lauraine E et al (2001) Predicting cancer-associated anaemia in patients receiving non-platinum chemotherapy: results of a retrospective survey. Eur J Cancer 37:1617–1623. https://doi.org/10.1016/s0959-8049(01)00169-1
Shitara K, Matsuo K, Oze I et al (2011) Meta-analysis of neutropenia or leukopenia as a prognostic factor in patients with malignant disease undergoing chemotherapy. Cancer Chemother Pharmacol 68:301–307. https://doi.org/10.1007/s00280-010-1487-6
Jinna S, Khandhar PB (2023) Thrombocytopenia. In: StatPearls. StatPearls Publishing, Treasure Island (FL)
Kuter DJ (2022) Treatment of chemotherapy-induced thrombocytopenia in patients with non-hematologic malignancies. Haematologica 107:1243–1263. https://doi.org/10.3324/haematol.2021.279512
Brasileiro LDA, Oliveira JMD, Castilho SRD (2021) Incidência E manejo da neutropenia em pacientes submetidas Ao Protocolo AC-T no tratamento adjuvante de câncer de mama. RSD 10:e48310616023. https://doi.org/10.33448/rsd-v10i6.16018
Schirrmacher V (2019) From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment (review). Int J Oncol 54:407–419. https://doi.org/10.3892/ijo.2018.4661
Barbosa G, Gelves LGV, Costa CMX et al (2022) Discovery of putative dual inhibitor of tubulin and EGFR by phenotypic Approach on LASSBio-1586 homologs. Pharmaceuticals (Basel) 15:913. https://doi.org/10.3390/ph15080913
Acknowledgements
The authors thank the Biotério of Centro Universitário Saúde ABC for providing the cell line from the Ehrlich ascites tumor used in this study.
Funding
This work was supported by the Brazilian National Council for Scientific and Technological Development (CNPq grant numbers 465249/2014-0, 306827/2023-9), the São Paulo Research Foundation (FAPESP grant numbers 2023/01502-1, 2018/12219-0), FAPERJ (grant number E-26/010.000090/2018), and the Coordination for the Improvement of Higher Education Personnel - finance code 001.
Author information
Authors and Affiliations
Contributions
Camila Chagas: Conceptualization, Formal analysis, Investigation, Methodology, Writing– original draft, Writing– review & editing. Jaqueline Vital Mansano: Formal analysis, Investigation, Methodology. Emerson Barbosa da Silva: Formal analysis, Investigation, Methodology, Writing– original draft. Giuliana Petri: Investigation, Methodology, Writing– original draft. Beatriz da Costa Aguiar Alves Reis: Writing– original draft. Maria Lúcia Schumacher: Writing– original draft. Paula Silvia Haddad: Formal analysis, Investigation, Writing– original draft. Edimar Cristiano Pereira: Formal analysis, Investigation, Writing– original draft. Tatiane Nassar Britos: Writing– original draft. Eliezer J. Barreiro: Resources. Lídia Moreira Lima: Resources. Fabio Furlan Ferreira: Resources, Software, Project administration, Writing– review & editing. Fernando Luiz Affonso Fonseca: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Writing– original draft, Writing - review & editing, Project administration, Resources.
Corresponding authors
Ethics declarations
Ethics approval
This work was registered and approved (# 12/2018) by the Commission on Ethics in the Use of Animals (CEUA-FMABC).
Informed consent
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chagas, C., Mansano, J.V., da Silva, E.B. et al. In vitro results with minimal blood toxicity of a combretastatin A4 analogue. Invest New Drugs (2024). https://doi.org/10.1007/s10637-024-01440-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10637-024-01440-4