Summary
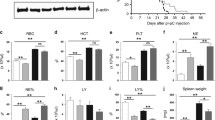
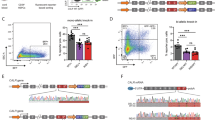
Background. Myeloproliferative neoplasms (MPN) are disorders characterized by an alteration at the hematopoietic stem cell (HSC) level, where the JAK2 mutation is the most common genetic alteration found in classic MPN (polycythemia vera, essential thrombocythemia, and primary myelofibrosis). We and others previously demonstrated that metformin reduced splenomegaly and platelets counts in peripheral blood in JAK2V617F pre-clinical MPN models, which highlighted the antineoplastic potential of biguanides for MPN treatment. Phenformin is a biguanide that has been used to treat diabetes, but was withdrawn due to its potential to cause lactic acidosis in patients. Aims. We herein aimed to investigate the effects of phenformin in MPN disease burden and stem cell function in Jak2V617F-knockin MPN mice. Results. In vitro phenformin treatment reduced cell viability and increased apoptosis in SET2 JAK2V67F cells. Long-term treatment with 40 mg/kg phenformin in Jak2V617F knockin mice increased the frequency of LSK, myeloid progenitors (MP), and multipotent progenitors (MPP) in the bone marrow. Phenformin treatment did not affect peripheral blood counts, spleen weight, megakaryocyte count, erythroid precursors frequency, or ex vivo clonogenic capacity. Ex vivo treatment of bone marrow cells from Jak2V617F knockin mice with phenformin did not affect hematologic parameters or engraftment in recipient mice. Conclusions. Phenformin increased the percentages of LSK, MP, and MPP populations, but did not reduce disease burden in Jak2V617F-knockin mice. Additional studies are necessary to further understand the effects of phenformin on early hematopoietic progenitors.




Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Swerdlow SH, Campo E, Harris NL et al (2017) WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues, 4a Edição. Lyon
DAMESHEK W (1951) Some speculations on the myeloproliferative syndromes. Blood 6:372–375
Baxter EJ, Scott LM, Campbell PJ et al (2005) Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365:1054–1061. https://doi.org/10.1016/S0140-6736(05)71142-9
Kralovics R, Passamonti F, Buser AS et al (2005) A Gain-of-Function Mutation of JAK2 in Myeloproliferative Disorders. N Engl J Med 352:1779–1790. https://doi.org/10.1056/NEJMoa051113
Levine RL, Wadleigh M, Cools J et al (2005) Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7:387–397. https://doi.org/10.1016/j.ccr.2005.03.023
James C, Ugo V, Le Couédic J-P et al (2005) A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 434:1144–1148. https://doi.org/10.1038/nature03546
Hubbard SR (2018) Mechanistic Insights into Regulation of JAK2 Tyrosine Kinase. Front Endocrinol (Lausanne) 8:361. https://doi.org/10.3389/fendo.2017.00361
Bailey CJ, Turner RC (1996) Metformin. N Engl J Med 334:574–579. https://doi.org/10.1056/NEJM199602293340906
Pollak M (2010) Metformin and Other Biguanides in Oncology: Advancing the Research Agenda. Cancer Prev Res 3:1060–1065. https://doi.org/10.1158/1940-6207.CAPR-10-0175
Neto EMR, Marques LARV, Ferreira MAD et al (2015) Metformina: Uma Revisão da Literatura. Saúde e Pesqui 8:355–362. https://doi.org/10.17765/2176-9206.2015V8N2P355-362
Dykens JA, Jamieson J, Marroquin L et al (2008) Biguanide-induced mitochondrial dysfunction yields increased lactate production and cytotoxicity of aerobically-poised HepG2 cells and human hepatocytes in vitro. Toxicol Appl Pharmacol 233:203–210. https://doi.org/10.1016/j.taap.2008.08.013
Kuntz EM, Baquero P, Michie AM et al (2017) Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells. Nat Med 23:1234–1240. https://doi.org/10.1038/nm.4399
Kawashima I, Kirito K (2016) Metformin inhibits JAK2V617F activity in MPN cells by activating AMPK and PP2A complexes containing the B56α subunit. Exp Hematol 44:1156-1165.e4. https://doi.org/10.1016/j.exphem.2016.08.005
Machado-Neto JA, Fenerich BA, Scopim-Ribeiro R et al (2018) Metformin exerts multitarget antileukemia activity in JAK2V617F-positive myeloproliferative neoplasms. Cell Death Dis 9:311. https://doi.org/10.1038/s41419-017-0256-4
Coelho-Silva JL, Bianco TM, Silva ABA et al (2019) Metformin Suppress Cellular and Molecular Processes Related to Maintenance and Proliferation of Myeloproliferative Neoplasm Stem Cell. Blood 134:1682–1682. https://doi.org/10.1182/blood-2019-132115
Orecchioni S, Reggiani F, Talarico G et al (2015) The biguanides metformin and phenformin inhibit angiogenesis, local and metastatic growth of breast cancer by targeting both neoplastic and microenvironment cells. Int J Cancer 136:E534–E544. https://doi.org/10.1002/ijc.29193
Somlyai G, Collins TQ, Meuillet EJ et al (2017) Structural homologies between phenformin, lipitor and gleevec aim the same metabolic oncotarget in leukemia and melanoma. Oncotarget 8:50187–50192. https://doi.org/10.18632/oncotarget.16238
Yuan P, Ito K, Perez-Lorenzo R et al (2013) Phenformin enhances the therapeutic benefit of BRAFV600E inhibition in melanoma. Proc Natl Acad Sci 110:18226–18231. https://doi.org/10.1073/pnas.1317577110
Hu S, Ouyang Q, Cheng Q et al (2018) Phenformin inhibits cell proliferation and induces cell apoptosis and autophagy in cholangiocarcinoma. Mol Med Rep 17:6028–6032. https://doi.org/10.3892/mmr.2018.8573
Guo Z, Zhao M, Howard EW et al (2017) Phenformin inhibits growth and epithelial-mesenchymal transition of ErbB2-overexpressing breast cancer cells through targeting the IGF1R pathway. Oncotarget 8. https://doi.org/10.18632/oncotarget.19466
Velez J, Pan R, Lee JTC et al (2016) Biguanides sensitize leukemia cells to ABT-737-induced apoptosis by inhibiting mitochondrial electron transport. Oncotarget 7:51435–51449. https://doi.org/10.18632/oncotarget.9843
Vara-Ciruelos D, Dandapani M, Russell FM et al (2019) Phenformin, But Not Metformin, Delays Development of T Cell Acute Lymphoblastic Leukemia/Lymphoma via Cell-Autonomous AMPK Activation. Cell Rep 27:690-698.e4. https://doi.org/10.1016/j.celrep.2019.03.067
Veiga SR, Ge X, Mercer CA et al (2018) Phenformin-Induced Mitochondrial Dysfunction Sensitizes Hepatocellular Carcinoma for Dual Inhibition of mTOR. Clin Cancer Res 24:3767–3780. https://doi.org/10.1158/1078-0432.CCR-18-0177
Segal ED, Yasmeen A, Beauchamp M-C et al (2011) Relevance of the OCT1 transporter to the antineoplastic effect of biguanides. Biochem Biophys Res Commun 414:694–699. https://doi.org/10.1016/j.bbrc.2011.09.134
Lamhonwah A-M, Tein I (2006) Novel localization of OCTN1, an organic cation/carnitine transporter, to mammalian mitochondria. Biochem Biophys Res Commun 345:1315–1325. https://doi.org/10.1016/j.bbrc.2006.05.026
McGuinness ME, Talbert RL (1993) Phenformin-Induced Lactic Acidosis: A Forgotten Adverse Drug Reaction. Ann Pharmacother 27:1183–1187. https://doi.org/10.1177/106002809302701004
WILLIAMS RH (1975) Farewell to Phenformin for Treating Diabetes Mellitus. Ann Intern Med 83:567. https://doi.org/10.7326/0003-4819-83-4-567
Nattrass M, Todd PG, Hinks L et al (1977) Comparative effects of phenformin, metformin and glibenclamide on metabolic rhythms in maturity-onset diabetics. Diabetologia 13:145–152. https://doi.org/10.1007/BF00745143
Baker NC, Ekins S, Williams AJ, Tropsha A (2018) A bibliometric review of drug repurposing. Drug Discov Today 23:661–672. https://doi.org/10.1016/j.drudis.2018.01.018
Wang Y, Meng Y, Zhang S et al (2018) Phenformin and metformin inhibit growth and migration of LN229 glioma cells in vitro and in vivo. Onco Targets Ther 11:6039–6048. https://doi.org/10.2147/OTT.S168981
Cook DE (1978) The effects of phenformin in normal vs. diabetic isolated perfused rat liver. Res Commun Chem Pathol Pharmacol 22:119–134
Dietze G, Wicklmayr M, Mehnert H et al (1978) Effect of phenformin on hepatic balances of gluconeogenic substrates in man. Diabetologia 14:243–248. https://doi.org/10.1007/BF01219423
Schlienger JL, Frick A, Marbach J et al (1979) Effects of biguanides on the intermediate metabolism of glucose in normal and portal-strictured rats. Diabete Metab 5:5–9
Shih Y-R, Kang H, Rao V et al (2017) In vivo engineering of bone tissues with hematopoietic functions and mixed chimerism. Proc Natl Acad Sci 114:5419–5424. https://doi.org/10.1073/pnas.1702576114
Busch K, Klapproth K, Barile M et al (2015) Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature 518:542–546. https://doi.org/10.1038/nature14242
Zhang Q-S, Tang W, Deater M et al (2016) Metformin improves defective hematopoiesis and delays tumor formation in Fanconi anemia mice. Blood 128:2774. https://doi.org/10.1182/BLOOD-2015-11-683490
Pollard JA, Furutani EM, Liu S et al (2021) Metformin for Treatment of Cytopenias in Children and Young Adults with Fanconi Anemia. Blood 138:1102–1102. https://doi.org/10.1182/blood-2021-153598
Uozumi K, Otsuka M, Ohno N et al (2000) Establishment and characterization of a new human megakaryoblastic cell line (SET-2) that spontaneously matures to megakaryocytes and produces platelet-like particles. Leukemia 14:142–152. https://doi.org/10.1038/sj.leu.2401608
Koulnis M, Pop R, Porpiglia E et al (2011) Identification and Analysis of Mouse Erythroid Progenitors using the CD71/TER119 Flow-cytometric Assay. J Vis Exp. https://doi.org/10.3791/2809
Challen GA, Boles N, Lin K-YK, Goodell MA (2009) Mouse hematopoietic stem cell identification and analysis. Cytom Part A 75A:14–24. https://doi.org/10.1002/cyto.a.20674
Acknowledgements
The authors would like to thank Dr Nicola Coran for English revision.
Funding
Funding for this work was supported in part by Fundação de Amparo à Pesquisa do Estado de São Paulo/São Paulo Research Foundation (FAPESP Grant #2013/08135–2), Conselho Nacional de Desenvolvimento Científico e Tecnológico/National Counsel of Technological and Scientific Development (CNPq; 2014/50947–7 [INCTC]; and 426633/2018–0), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Contributions
A.B.A-S designed, executed and analyzed the experiments and prepared the manuscript. B.A.F., N.P.F. participated in experiments and prepared the manuscript. J.C.F provided inputs and participated in experiments. J.L.C-S participated and designed experiments. D.A.P-M., T.M.B., P.S.S. participated in flow cytometry experiments and data analysis. E.M.R. and L.L.F-P provided inputs on experimental designed and interpretation of the data. F.C. participated in histopathology experiments. J.A.M–N., designed, analyzed, provided inputs and prepared the manuscript. F.T supervised and participated in overall design of study, experiments and analyses. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving animals were approved and were following recommendations from Ethical Committee for Animal Research from University of São Paulo at Ribeirão Preto Medical School (183/2018) and followed by Grimace scale for assessing pain in mice.
Informed consent
Not applicable.
Consent for publication
Not applicable.
Research involving Human Participants and/or Animals
This study did not involved human participants. Studies involving animals were approved by Ethical Committee for Animal Research from University of São Paulo at Ribeirão Preto Medical School (183/2018).
Conflict of interests
Antônio Bruno Alves-Silva declares that he has no competing interests. Bruna Alves Fenerich declares that she has no competing interests. Natasha Peixoto Fonseca declares that she has no competing interests. Jaqueline Cristina Fernandes declares that she has no competing interests. Juan Luiz Coelho-Silva declares that he has no competing interests. Diego Antonio Pereira-Martins declares that he has no competing interests. Thiago Mantello Bianco declares that he has no competing interests. Priscila Santos Scheucher declares that she has no competing interests. Eduardo Magalhães Rego declares that he has no competing interests. Fernando Chahud declares that he has no competing interests. João Agostinho Machado-Neto declares that he has no competing interests. Lorena Lôbo Figueiredo-Pontes declares that she has no competing interests. Fabiola Traina declares that she has no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alves-Silva, A.B., Fenerich, B.A., Fonseca, N.P. et al. Phenformin increases early hematopoietic progenitors in the Jak2V617F murine model. Invest New Drugs 40, 576–585 (2022). https://doi.org/10.1007/s10637-022-01212-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01212-y