Abstract
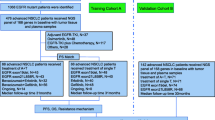
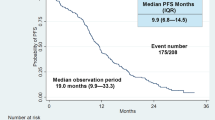
Background and objective. Osimertinib as first-line treatment for patients with non-small cell lung cancer (NSCLC) harboring epidermal growth factor (EGFR) mutations remains controversial. Sequential EGFR-tyrosine kinase inhibitor (TKI) might be superior to the first line osimertinib in patients at risk of developing acquired T790M mutations. Methods. We enrolled consecutive patients with EGFR-mutated (deletion 19 or L858R) advanced NSCLC treated with first-line drugs and evaluated predictive markers using classification and regression tree (CART) for the detection of T790M mutations based on patient backgrounds prior to initial treatment. Results. Patients without acquired T790M mutations had worse outcomes than those with T790M mutations (median OS: 798 days vs. not reached; HR: 2.70; P < 0.001). CART identified three distinct groups based on variables associated with acquired T790M mutations (age, CYF, WBC, liver metastasis, and LDH; AUROC: 0.77). Based on certain variables, CART identified three distinct groups in deletion 19 (albumin, LDH, bone metastasis, pleural effusion, and WBC; AUROC: 0.81) and two distinct groups in L858R (age, CEA, and ALP; AUROC: 0.80). The T790M detection frequencies after TKI resistance of afatinib and first-generation EGFR-TKIs were similar (35.3% vs. 37.4%, P = 0.933). Afatinib demonstrated longer PFS (398 vs. 279 days; HR: 0.67; P = 0.004) and OS (1053 vs. 956 days; HR: 0.68; P = 0.051) than first-generation EGFR-TKIs. Conclusion. Identification of patients at risk of acquiring T790M mutations after EGFR-TKI failure may aid in choice of first-line EGFR-TKI. Furthermore, afatinib may be the more effective 1st-line EGFR-TKI treatment for patients at risk of developing T790M as initial EGFR-TKI resistance.




Similar content being viewed by others
Data availability
The data that support the findings of this study are openly available in University hospital Medical Information Network at https://www.umin.ac.jp, reference number UMIN000041474.
References
Park K, Tan EH, O’Byrne K, Zhang L, Boyer M, Mok T et al (2016) Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 17:577–589
Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S et al (2017) Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 18:1454–1466
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH et al (2018) Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 378:113–125
Akamatsu H, Ninomiya K, Kenmotsu H, Morise M, Daga H, Goto Y et al (2019) The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV. Int J Clin Oncol 24:731–770
Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ et al (2014) AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 4:1046–1061
Arcila ME, Oxnard GR, Nafa K, Riely GJ, Solomon SB, Zakowski MF et al (2011) Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res 17:1169–1180
Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, Kris MG et al (2011) Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 17:1616–1622
Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS et al (2017) Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 376:629–640
Schoenfeld AJ, Chan JM, Kubota D, Sato H, Rizvi H, Daneshbod Y et al (2020) Tumor Analyses Reveal Squamous Transformation and Off-Target Alterations As Early Resistance Mechanisms to First-line Osimertinib in EGFR-Mutant Lung Cancer. Clin Cancer Res 26:2654–2663
Fernandes MGO, Sousa C, Jacob M, Almeida L, Santos V, Araújo D et al (2021) Resistance Profile of Osimertinib in Pre-treated Patients With EGFR T790M-Mutated Non-small Cell Lung Cancer Front Oncol 11:602924
Oxnard GR, Hu Y, Mileham KF, Husain H, Costa DB, Tracy P et al (2018) Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol 4:1527–1534
Gray JE, Okamoto I, Sriuranpong V, Vansteenkiste J, Imamura F, Lee JS et al (2019) Tissue and Plasma EGFR Mutation Analysis in the FLAURA Trial: Osimertinib versus Comparator EGFR Tyrosine Kinase Inhibitor as First-Line Treatment in Patients with EGFR-Mutated Advanced Non-Small Cell Lung Cancer. Clin Cancer Res 25:6644–6652
Seto T, Nogami N, Yamamoto N, Atagi S, Tashiro N, Yoshimura Y et al (2018) Real-World EGFR T790M Testing in Advanced Non-Small-Cell Lung Cancer: A Prospective Observational Study in Japan. Oncol Ther 6:203–215
Ninomaru T, Hata A, Kokan C, Okada H, Tomimatsu H, Ishida J (2021) Higher osimertinib introduction rate achieved by multiple repeated rebiopsy after acquired resistance to first/second generation EGFR-TKIs. Thorac Cancer 12:746–751
Ohe Y, Imamura F, Nogami N, Okamoto I, Kurata T, Kato T et al (2019) Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn J Clin Oncol 49:29–36
Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M (2019) Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer 121:725–737
Yamamoto N, Mera T, Märten A, Hochmair MJ (2020) Observational Study of Sequential Afatinib and Osimertinib in EGFR Mutation-Positive NSCLC: Patients Treated with a 40-mg Starting Dose of Afatinib. Adv Ther 37:759–769
Ichihara E, Hotta K, Kubo T, Higashionna T, Ninomiya K, Ohashi K et al (2018) Clinical significance of repeat rebiopsy in detecting the EGFR T790M secondary mutation in patients with non-small cell lung cancer. Oncotarget 9:29525–29531
Mountzios G, Koumarianou A, Bokas A, Mavroudis D, Samantas E, Fergadis EG et al (2021) A Real-World, Observational, Prospective Study to Assess the Molecular Epidemiology of Epidermal Growth Factor Receptor (EGFR) Mutations upon Progression on or after First-Line Therapy with a First- or Second-Generation EGFR Tyrosine Kinase Inhibitor in EGFR Mutation-Positive Locally Advanced or Metastatic Non-Small Cell Lung Cancer: The “LUNGFUL” Study. Cancers (Basel) 13:3172
Hochmair MJ, Buder A, Schwab S, Burghuber OC, Prosch H, Hilbe W et al (2019) Liquid-Biopsy-Based Identification of EGFR T790M Mutation-Mediated Resistance to Afatinib Treatment in Patients with Advanced EGFR Mutation-Positive NSCLC, and Subsequent Response to Osimertinib. Target Oncol 14:75–83
Buder A, Hochmair MJ, Schwab S, Bundalo T, Schenk P, Errhalt P et al (2018) Cell-Free Plasma DNA-Guided Treatment With Osimertinib in Patients With Advanced EGFR-Mutated NSCLC. J Thorac Oncol 13:821–830
Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W (2003) Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med 26:172–181
Matsuo N, Azuma K, Sakai K, Hattori S, Kawahara A, Ishii H et al (2016) Association of EGFR Exon 19 Deletion and EGFR-TKI Treatment Duration with Frequency of T790M Mutation in EGFR-Mutant Lung Cancer Patients. Sci Rep 6:36458
Nosaki K, Satouchi M, Kurata T, Yoshida T, Okamoto I, Katakami N et al (2016) Re-biopsy status among non-small cell lung cancer patients in Japan: A retrospective study. Lung Cancer 101:1–8
Carey KD, Garton AJ, Romero MS, Kahler J, Thomson S, Ross S et al (2006) Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res 66:8163–8171
Zhu JQ, Zhong WZ, Zhang GC, Li R, Zhang XC, Guo AL et al (2008) Better survival with EGFR exon 19 than exon 21 mutations in gefitinib-treated non-small cell lung cancer patients is due to differential inhibition of downstream signals. Cancer Lett 265:307–317
Cho J, Chen L, Sangji N, Okabe T, Yonesaka K, Francis JM et al (2013) Cetuximab response of lung cancer-derived EGF receptor mutants is associated with asymmetric dimerization. Cancer Res 73:6770–6779
Li WQ, Cui JW (2020) Non-small cell lung cancer patients with ex19del or exon 21 L858R mutation: distinct mechanisms, different efficacies to treatments. J Cancer Res Clin Oncol 146:2329–2338
Acknowledgements
This study was funded by Boehringer Ingelheim. We thank the staff, data manager and other support staff at all investigational sites.
Funding
This study was funded by Boehringer Ingelheim.
Author information
Authors and Affiliations
Contributions
(I) Conception and design: M.T., K.F., A.T., and S.T., (II) Administrative support: M.T., K.F., A.T., and S.T., (III) Provision of study materials or patients: All authors,, (IV) Collection and assembly of data: All authors, (V) Data analysis and interpretation: M.T., K.F., H.S., A.T., and S.T., (VI) Manuscript writing: All authors,
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethical review board or institutional review board of each participating institute, and main institutional review board (IRB) approval for this study was obtained from the Medical Research Ethics Committee of Osaka International Cancer Institute.
Informed consent
Informed consent was not required owing to the retrospective nature of the study, and an opt-out method was utilized so that patients and their families could refuse to participate in the study.
Consent for publication
All authors approved final manuscript.
Research involving Human Participants and/or Animals
Research involving Human Participants.
Conflict of interests
M.T. has grants from Ono Pharmaceutical, Bristol-Myers Squibb, and BoehringerIngelheim, and payment or honoraria from AstraZeneca, Ono Pharmaceutical, Bristol-Myers Squibb, Taiho Pharmaceutical, Chugai Pharmaceutical, MSD, BoehringerIngelheim, Eli Lilly, Kyowa Kirin, Pfizer, and Asahi Kasei Pharmaceutical. H.S. has payment or honoraria from Chugai Pharmaceutical and AstraZeneca. A.T. has grants from AstraZeneca, and payment or honoraria from AstraZeneca, Ono Pharmaceutical, Bristol-Myers Squibb, Taiho Pharmaceutical, Chugai Pharmaceutical, MSD, BoehringerIngelheim, Eli Lilly, Kyowa Kirin, Pfizer, and Kissei, and advisory board from AstraZeneca, Pfizer, amd Ono Pharmaceutical. Y.S. has payment or honoraria from Chugai Pharmaceutical and AstraZeneca. M.K. has payment or honoraria from AstraZeneca, Ono Pharmaceutical, Shionogi Pharmaceutical, Chugai Pharmaceutical, MSD, BoehringerIngelheim, and Eli Lilly. T.S. has payment or honoraria from Kyorin Pharmaceutical, Ono Pharmaceutical, Daiichi Sankyo, Chugai Pharmaceutical, AstraZeneca, and Novartis Pharmaceutical. F.D. has grants from AstraZeneca, and Boehringer Ingelheim, and payment or honoraria from AstraZeneca, Ono Pharmaceutical, Bristol-Myers Squibb, Taiho Pharmaceutical, Chugai Pharmaceutical, MSD, BoehringerIngelheim, and Eli Lilly. Other co-authors have no COI. This study was approved by the ethical review board or institutional review board of each participating institute. Informed consent was not required owing to the retrospective nature of the study, and an opt-out method was utilized so that patients and their families could refuse to participate in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tamiya, M., Fujikawa, K., Suzuki, H. et al. Classification and regression tree for estimating predictive markers to detect T790M mutations after acquired resistance to first line EGFR-TKI: HOPE-002. Invest New Drugs 40, 361–369 (2022). https://doi.org/10.1007/s10637-021-01203-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01203-5