Summary
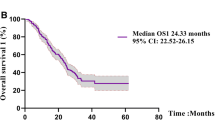
Objective. The clinical outcomes of poor performance status (PS) patients with epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC) who are treated with osimertinib as a first-line treatment have not been sufficiently evaluated. This study aimed to assess the efficacy and safety of osimertinib in chemotherapy-naive and poor PS (2 or more) patients with NSCLC harboring sensitive EGFR mutations. Materials and Methods. We assessed the clinical effects of osimertinib as a first-line treatment for patients with poor PS NSCLC with an exon 19 deletion or exon 21 L858R mutation in EGFR. All patients were administered osimertinib (80 mg/day) as the initial treatment. Results. Sixteen patients (nine women and seven men) who were treated between August 2018 and July 2021 were included in this study; their median age was 78 years. The overall objective response rate was 56.3%. The median progression-free survival (PFS) of the entire patient population was 10.5 months and the PS score improved in 8 of 16 patients (50%). The most common adverse event was acneiform rash (42%), followed by diarrhea (36%) and paronychia (36%); none of these were of grade ≥ 3. Interstitial lung disease occurred in 2 patients (12.5%); however, no treatment-related deaths occurred. Conclusion. Considering the findings of this study, osimertinib appears to be an effective and safe treatment option for patients with poor PS and advanced NSCLC harboring sensitive EGFR mutations. To obtain conclusive results, further studies with larger cohorts are warranted.




Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA (2008) Non-small-cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83:584–594. https://doi.org/10.4065/83.5.584
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29. https://doi.org/10.3322/caac.20138
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med 350:2129–2139. https://doi.org/10.1056/NEJMoa040938
Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, Fujisawa T, Feng Z, Roth JA, Herz J, Minna JD, Gazdar AF (2005) Clinical andbiological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 97:339–346. https://doi.org/10.1093/jnci/dji055
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T, North-East Japan Study Group (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388. https://doi.org/10.1056/NEJMoa0909530
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M, West Japan Oncology Group (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 11:121–128. https://doi.org/10.1016/S1470-2045(09)70364-X
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, Longo F, Moran T, Insa A, De Marinis F, Corre R, Bover I, Illiano A, Dansin E, de Castro J, Milella M, Reguart N, Altavilla G, Jimenez U, Provencio M, Moreno MA, Terrasa J, Muñoz-Langa J, Valdivia J, Isla D, Domine M, Molinier O, Mazieres J, Baize N, Garcia-Campelo R, Robinet G, Rodriguez-Abreu D, Lopez-Vivanco G, Gebbia V, Ferrera-Delgado L, Bombaron P, Bernabe R, Bearz A, Artal A, Cortesi E, Rolfo C, Sanchez-Ronco M, Drozdowskyj A, Queralt C, de Aguirre I, Ramirez JL, Sanchez JJ, Molina MA, Taron M, Paz-Ares L (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-smallcell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13:239–246. https://doi.org/10.1016/S1470-2045(11)70393-X
Zhou C, Wu YL, Chen G, Feng J, Liu X, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, Hu C, Luo Y, Chen L, Ye M, Huang J, Zhi X, Zhang Y, Xiu Q, Ma J, Zhang L, You C (2011) Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, openlabel, randomised, phase 3 study. Lancet Oncol 12:735–742. https://doi.org/10.1016/S1470-2045(11)70184-X
Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, Su WC, Bennouna J, Kato T, Gorbunova V, Lee KH, Shah R, Massey D, Zazulina V, Shahidi M, Schuler M (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31:3327–3334. https://doi.org/10.1200/JCO.2012.44.2806
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, Li W, Hou M, Shi JH, Lee KY, Xu CR, Massey D, Kim M, Shi Y, Geater SL (2014) Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol 15:213–222. https://doi.org/10.1016/S1470-2045(13)70604-1
Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, Migliorino MR, Pluzanski A, Sbar EI, Wang T, White JL, Nadanaciva S, Sandin R, Mok TS (2017) Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutationpositive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol 18:1454–1466. https://doi.org/10.1016/S1470-2045(17)30608-3
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, Okamoto I, Zhou C, Cho BC, Cheng Y, Cho EK, Voon PJ, Planchard D, Su WC, Gray JE, Lee SM, Hodge R, Marotti M, Rukazenkov Y, Ramalingam SS, FLAURA Investigators (2018) Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378:113–125 and methods. https://doi.org/10.1056/NEJMoa1713137
Igawa S, Ono T, Kasajima M, Kusuhara S, Otani S, Fukui T, Yokoba M, Kubota M, Katagiri M, Mitsufuji H, Sasaki J, Naoki K (2020) Real-world assessment of afatinib for patients with EGFR-positive non-small cell lung cancer. Investig New Drugs 38:1906–1914. https://doi.org/10.1007/s10637-020-00948-9
Ono T, Igawa S, Kurahayashi S, Okuma Y, Sugimoto A, Kusuhara S, Ozawa T, Fukui T, Sasaki J, Mitsufuji H, Yokoba M, Kubota M, Katagiri M, Naoki K (2020) Impact of neutrophil-to-lymphocyte ratio in patients with EGFR-mutant NSCLC treated with tyrosine kinase inhibitors. Investig New Drugs 38:885–893. https://doi.org/10.1007/s10637-020-00919-0 (Epub 2020 Mar 10 PMID: 32157598)
Igawa S, Ryuge S, Ichinoe M, Nakashima H, Otani S, Nakahara Y, Fukui T, Sasaki J, Kubota M, Katagiri M, Murakumo Y, Satoh Y, Sato Y, Masuda N (2017) Impact of EGFR-tyrosine kinase inhibitors on postoperative recurrent non-small-cell lung cancer harboring EGFR mutations. Oncol Res Treat 40:7–13. https://doi.org/10.1159/000455147
Inoue A, Kobayashi K, Usui K, Maemondo M, Okinaga S, Mikami I, Ando M, Yamazaki K, Saijo Y, Gemma A, Miyazawa H, Tanaka T, Ikebuchi K, Nukiwa T, Morita S, Hagiwara K, North East Japan Gefitinib Study Group (2009) First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. North East Japan gefitinib Study Group. J Clin Oncol 27:1394–1400. https://doi.org/10.1200/JCO.2008.18.7658
Okuma Y, Hosomi Y, Nagamata M, Yamada Y, Sekihara K, Kato K, Hishima T, Okamura T (2013) Clinical outcomes after first-line EGFR inhibitor treatment for patients with NSCLC, EGFR mutation, and poor performance status. Anticancer Res 33:5057–5064
Wu CE, Chang CF, Huang CY, Yang CT, Kuo CS, Hsu PC, Chang JW (2021) Feasibility and effectiveness of afatinib for poor performance status patients with EGFR-mutation-positive non-small-cell lung cancer: A retrospective cohort study. BMC Cancer 21:859. https://doi.org/10.1186/s12885-021-08587-w
Nishii Y, Hataji O, Ito K, Watanabe F, Kobayashi T, D’Alessandro-Gabazza C, Toda M, Taguchi O, Yamamoto N, Gabazza EC (2018) Efficacy of osimertinib in a patient with non-small cell lung cancer harboring epithelial growth factor receptor exon 19 deletion/T790M mutation, with poor performance status. Mol Clin Oncol 8:246–249. https://doi.org/10.3892/mco.2017.1522
Nakashima K, Kimura M, Akamatsu H, Daga H, Imai H, Taira T, Ko R, Hisamatsu Y, Nishino K, Sugimoto T, Miyashita Y, Takahashi T et al (2019) Osimertinib for patients with EGFR T790M mutation-positive non-small-cell lung cancer and a poor performance status. Jpn J Clin Oncol 49:671–675. https://doi.org/10.1093/jjco/hyz041
Nakashima K, Ozawa Y, Daga H, Imai H, Tamiya M, Tokito T, Kawamura T, Akamatsu H, Tsuboguchi Y, Takahashi T, Yamamoto N, Mori K, Murakami H (2020) Osimertinib for patients with poor performance status and EGFR T790M mutation-positive advanced non-small cell lung cancer: A phase II clinical trial. Investig New Drugs 38:1854–1861. https://doi.org/10.1007/s10637-020-00943-0
Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E (2012) Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 62:220–241. https://doi.org/10.3322/caac.21149
Ohe Y, Imamura F, Nogami N, Okamoto I, Kurata T, Kato T, Sugawara S, Ramalingam SS, Uchida H, Hodge R, Vowler SL, Walding A, Nakagawa K (2019) Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn J Clin Oncol 49:29–36. https://doi.org/10.1093/jjco/hyy179
Gemma A, Kusumoto M, Sakai F, Endo M, Kato T, Saito Y, Baba T, Sata M, Yamaguchi O, Yabuki Y, Nogi Y, Jinushi M, Sakamoto K, Sugeno M, Tamura R, Tokimoto T, Ohe Y (2020) Real-world evaluation of factors for interstitial lung disease incidence and radiologic characteristics in patients with EGFR T790M-positive NSCLC treated with Osimertinib in Japan. J Thorac Oncol 15:1893–1906. https://doi.org/10.1016/j.jtho.2020.08.025
Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, Lee CK, Sebastian M, Templeton A, Mann H, Marotti M, Ghiorghiu S, Papadimitrakopoulou VA, AURA3 Investigators (2017) Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med 376:629-640. https://doi.org/10.1056/NEJMoa1612674
Yang JC-H, Ahn MJ, Kim DW, Ramalingam SS, Sequist LV, Su WC, Kim SW, Kim JH, Planchard D, Felip E, Blackhall F, Haggstrom D, Yoh K, Novello S, Gold K, Hirashima T, Lin CC, Mann H, Cantarini M, Ghiorghiu S, Jänne PA (2017) Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol 35:1288–1296. https://doi.org/10.1200/JCO.2016.70.3223
Acknowledgements
We gratefully thank the staff members of the Department of Respiratory Medicine, Kitasato University School of Medicine, for their suggestions and assistance.
Funding
There was no funding to declare.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Data collection was performed by all authors, and analysis was performed by SI and MK. The first draft of the manuscript was written by SI and KN. All authors commented on versions of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Ethics Review Board of Kitasato University Hospital. All patients provided written informed consent prior to enrollment.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All authors the study gave consent to publication of this study.
Research involving human participants and/or animals
Not applicable.
Conflict of interests
There was no competing interest to declare. The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Igawa, S., Fukui, T., Kasajima, M. et al. First-line osimertinib for poor performance status patients with EGFR mutation-positive non-small cell lung cancer: A prospective observational study. Invest New Drugs 40, 430–437 (2022). https://doi.org/10.1007/s10637-021-01195-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01195-2