Summary
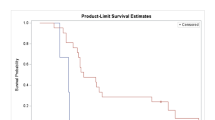
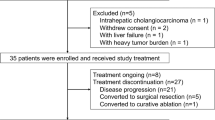
Introduction. We investigated the safety and efficacy of a pegylated arginase (PEG-BCT-100) in combination with chemotherapy (oxaliplatin and capecitabine) [PACOX] in advanced HCC patients. Methods. This was a single centre phase 1 trial to assess the safety and tolerability of PACOX. All the enrolled subjects received treatment in 3-weekly cycles: intravenous PEG-BCT-100 2.7 mg/kg on days 1, 8 and 15 of each cycle; oral capecitabine 1000 mg/m2 twice daily on day 1–14 of each cycle and intravenous oxaliplatin on day 1. Three dose levels of oxaliplatin (85 mg/m2, 100 mg/m2 or 130 mg/m2) were studied to define the maximum tolerated dose (MTD). Adverse events (AEs), efficacy by RECIST v1.1, time to progression (TTP), progression-free survival (PFS) and overall survival (OS) were studied. Results. Seventeen patients were enrolled at 3 dose levels of oxaliplatin: 85 mg/m2 (8 patients), 100 mg/m2 (3 patients), and 130 mg/m2 (6 patients). The median age was 55 years; all had had locoregional chemotherapy or targeted therapy such as sorafenib, but no systemic chemotherapy. The most common AEs were nausea (82%), injection site reaction (76%), palmar-plantar erythrodysesthesia (59%), oral mucositis (53%) and vomiting (53%). There was no dose-limiting toxicity (DLT). Median duration on study was 8 weeks overall. In 14 evaluable cases, one achieved partial response (PR), 4 had stable disease (SD); disease control rate was 36%; most responses were observed in the 130 mg/m2 cohort with 1 PR and 2 SDs. Median TTP and PFS were both 7.0 weeks. Overall median OS was 10.7 months; the median OS was not reached at 19.4 months of follow-up in the 130 mg/m2 cohort. Conclusion. The PACOX regimen demonstrated good anti-cancer activity and survival advantage in advanced pre-treated HCC with favourable safety profile. It warrants further phase II/III studies.



Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J et al (2013) Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol 31:3501–3508
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc J-F et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Cheng A-L, Kang Y-K, Chen Z, Tsao C-J, Qin S, Kim JS et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10:25–34
Yamashita T, Kudo M, Ikeda K, Izumi N, Tateishi R, Ikeda M et al (2020) REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol 55:113–122
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y et al (2021) IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). JCO Wolters Kluwer 39:267–267
Scott L, Lamb J, Smith S, Wheatley DN (2000) Single amino acid (arginine) deprivation: rapid and selective death of cultured transformed and malignant cells. Br J Cancer 83:800–810
Chan SL, Cheng PNM, Liu AM, Chan LL, Li L, Chu CM et al (2021) A phase II clinical study on the efficacy and predictive biomarker of pegylated recombinant arginase on hepatocellular carcinoma. Invest New Drugs [Internet]. 2021 [cited 2021 Jun 8]; Available from: https://link.springer.com/https://doi.org/10.1007/s10637-021-01111-8
Yang T-S, Lu S-N, Chao Y, Sheen I-S, Lin C-C, Wang T-E et al (2010) A randomised phase II study of pegylated arginine deiminase (ADI-PEG 20) in Asian advanced hepatocellular carcinoma patients. Br J Cancer 103:954–960
Abou-Alfa GK, Qin S, Ryoo B-Y, Lu S-N, Yen C-J, Feng Y-H et al (2018) Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann Oncol 29:1402–1408
Yau T, Cheng PN, Chan P, Chan W, Chen L, Yuen J et al (2013) A phase 1 dose-escalating study of pegylated recombinant human arginase 1 (Peg-rhArg1) in patients with advanced hepatocellular carcinoma. Invest New Drugs 31:99–107
Yau T, Cheng PN, Chan P, Chen L, Yuen J, Pang R et al (2015) Preliminary efficacy, safety, pharmacokinetics, pharmacodynamics and quality of life study of pegylated recombinant human arginase 1 in patients with advanced hepatocellular carcinoma. Invest New Drugs 33:496–504
De Santo C, Cheng P, Beggs A, Egan S, Bessudo A, Mussai F (2018) Metabolic therapy with PEG-arginase induces a sustained complete remission in immunotherapy-resistant melanoma. J Hematol Oncol 11:68
Cheng PN-M, Lam T-L, Lam W-M, Tsui S-M, Cheng AW-M, Lo W-H et al (2007) Pegylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res 67:309–17
Szlosarek PW, Steele JP, Nolan L, Gilligan D, Taylor P, Spicer J et al (2017) Arginine Deprivation With Pegylated Arginine Deiminase in Patients With Argininosuccinate Synthetase 1-Deficient Malignant Pleural Mesothelioma: A Randomized Clinical Trial. JAMA Oncol 3:58–66
Beddowes E, Spicer J, Chan PY, Khadeir R, Corbacho JG, Repana D et al (2017) Phase 1 Dose-Escalation Study of Pegylated Arginine Deiminase, Cisplatin, and Pemetrexed in Patients With Argininosuccinate Synthetase 1-Deficient Thoracic Cancers. J Clin Oncol 35:1778–1785
Hall PE, Lewis R, Syed N, Shaffer R, Evanson J, Ellis S et al (2019) A Phase I Study of Pegylated Arginine Deiminase (Pegargiminase), Cisplatin, and Pemetrexed in Argininosuccinate Synthetase 1-Deficient Recurrent High-grade Glioma. Clin Cancer Res 25:2708–2716
Yau T, Kang Y-K, Kim T-Y, El-Khoueiry AB, Santoro A, Sangro B et al (2020) Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol
Funding
Bio-Cancer Treatment International Ltd. and HKSARG Innovation and Technology Fund (project reference UIM/257).
Author information
Authors and Affiliations
Contributions
TY and PC designed the study, performed the research, and wrote the manuscript. AL wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Approval was obtained from the ethics committee.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publication
All authors of the study gave consent to publication of this study.
Conflicts of interest
PC and AL are employees of Bio-Cancer Treatment International Ltd.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yau, T., Cheng, P.N.M., Chiu, J. et al. A phase 1 study of pegylated recombinant arginase (PEG-BCT-100) in combination with systemic chemotherapy (capecitabine and oxaliplatin)[PACOX] in advanced hepatocellular carcinoma patients. Invest New Drugs 40, 314–321 (2022). https://doi.org/10.1007/s10637-021-01178-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01178-3