Summary
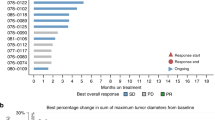
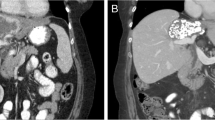
Background Biliary tract cancers (BTC) are rare, chemo resistant and are associated with a poor prognosis. Preclinical and early clinical work had demonstrated interesting anti-tumor activity from targeting fibroblast growth factor receptor (FGFR) pathway. We hypothesized that ponatinib, a multi-targeted tyrosine kinase inhibitor with activity against FGFR, would be active in BTC patients with FGFR alterations. Methods This was a multi-center, single institution pilot study of ponatinib in patients with advanced, refractory BTC with FGFR alterations. The primary end point was overall response rate, with secondary points of overall survival (OS), progression-free survival (PFS) and Health Related Quality of Life (HRQoL) assessment. Results Twelve patients were enrolled prior to early termination of the trial. Partial responses were observed in 1 from 12 patients. Median PFS was 2.4 months and median OS was 15.7 months. All observed toxicities were manageable and reversible. Toxicities were mild, with lymphopenia (75%), rash (63%) and fatigue (50%) being the most frequent. No significant detriment in global QoL was observed. Conclusions Ponatinib as a single agent in FGFR altered BTC is tolerable with limited clinical activity. This is the first report of prospective assessment of FGFR inhibition in BTC using ponatinib, and the first study to report its effect on HRQoL. Further development of ponatinib will involve correlative studies to better refine patient selection, focus on combinations with other molecular targeted agents, conventional cytotoxic chemotherapy, and studies to better understand mechanisms of treatment resistance.


Similar content being viewed by others
Data availability
All data and materials are available for verification as needed.
Code availability
Not applicable.
References
Torre LA, Siegel RL, Islami F, Bray F, Jemal A (2018) Worldwide burden of and trends in mortality from gallbladder and other biliary tract cancers. Clin Gastroenterol Hepatol 16(3):427–437
Jarnagin WR, Ruo L, Little SA et al (2003) Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer 98:1689–1700
Valle J, Wasan H, Palmer DH et al (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–1281
Lamarca A, Palmer DH, Wasan HS et al (2021) Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol 22(5):690–701
Paule B, Herelle MO, Rage E et al (2007) Cetuximab plus gemcitabine-oxaliplatin (GEMOX) in patients with refractory advanced intrahepatic cholangiocarcinomas. Oncology 72:105–110
Suzuki E, Ikeda M, Okusaka T et al (2013) A multicenter phase II study of S-1 for gemcitabine-refractory biliary tract cancer. Cancer Chemother Pharmacol 71:1141–1146
Bridgewater J, Palmer D, Cunningham D et al (2013) Outcome of second-line chemotherapy for biliary tract cancer. Eur J Cancer 49:1511
Croitoru A, Gramaticu I, Dinu I et al (2012) Fluoropyrimidines plus cisplatin versus gemcitabine/gemcitabine plus cisplatin in locally advanced and metastatic biliary tract carcinoma - a retrospective study. J Gastrointestin Liver Dis 21:277–284
Fornaro L, Vivaldi C, Cereda S et al (2015) Second-line chemotherapy in advanced biliary cancer progressed to first-line platinum-gemcitabine combination: a multicenter survey and pooled analysis with published data. J Exp Clin Cancer Res 34:156
Sasaki T, Isayama H, Nakai Y et al (2013) A pilot study of salvage irinotecan monotherapy for advanced biliary tract cancer. Anticancer Res 33:2619–2622
Belov AA, Mohammadi M (2013) Molecular mechanisms of fibroblast growth factor signaling in physiology and pathology. Cold Spring Harb Perspect Biol 5
Churi CR, Shroff R, Wang Y et al (2014) Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One 9:e115383
Javle M, Bekaii-Saab T, Jain A et al (2016) Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 122:3838–3847
Borad MJ, Champion MD, Egan JB et al (2014) Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet 10:e1004135
Ross JS, Wang K, Gay L et al (2014) New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist 19:235–242
Sia D, Losic B, Moeini A et al (2015) Massive parallel sequencing uncovers actionable FGFR2-PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma. Nat Commun 6:6087
Arai Y, Totoki Y, Hosoda F et al (2014) Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology 59:1427–1434
Wu YM, Su F, Kalyana-Sundaram S et al (2013) Identification of targetable FGFR gene fusions in diverse cancers. Cancer Discov 3:636–647
Graham RP, Barr Fritcher EG, Pestova E et al (2014) Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol 45:1630–1638
Kouhara H, Hadari YR, Spivak-Kroizman T et al (1997) A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 89:693–702
Abou-Alfa GK, Sahai V, Hollebecque A et al (2020) Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol 21(5):671–684
Javle M, Lowery M, Shroff RT et al (2018) Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol 36(3):276
Bridgewater J, Meric-Bernstam F, Hollebecque A et al (2020) Efficacy and safety of futibatinib in intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 fusions/other rearrangements: Subgroup analyses of a phase II study (FOENIX-CCA2). Ann Oncol 31:S261–S262
Gozgit JM, Wong MJ, Moran L et al (2012) Ponatinib (AP24534), a multitargeted pan-FGFR inhibitor with activity in multiple FGFR-amplified or mutated cancer models. Mol Cancer Ther 11:690–699
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Oken MM, Creech RH, Tormey DC et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Common Terminology Criteria for Adverse Events (CTCAE) (2021). In: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed on July 2021
Kaupp-Roberts SD, Yadegarfar G, Friend E et al (2016) Validation of the EORTC QLQ-BIL21 questionnaire for measuring quality of life in patients with cholangiocarcinoma and cancer of the gallbladder. Br J Cancer 115:1032–1038
Friend E, Yadegarfar G, Byrne C et al (2011) Development of a questionnaire (EORTC module) to measure quality of life in patients with cholangiocarcinoma and gallbladder cancer, the EORTC QLQ-BIL21. Br J Cancer 104:587–592
Chren MM, Lasek RJ, Sahay AP, Sands LP (2001) Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cut Med Surg 5(2):105–110
Atherton PJ, Halyard MY, Sloan MJ et al (2013) Assessment of patient-reported measures of bowel function before and after pelvic radiotherapy: an ancillary study of the North Central Cancer Treatment Group study N00CA.". Supp Care Cancer 21(4):1193–1199
Arora M, Bogenberger JM, Abdelrahman A et al (2020) Evaluation of NUC-1031: A first-in-class ProTide in biliary tract cancer.". Cancer chemother pharmacol 85:1063–1078
Uson Junior PLS, Bogenberger J, Borad MJ (2020) Advances in the treatment of biliary tract cancers. Curr opinion gastroenterol 36(2):85–89
Cleary JM, Iyer G, Oh DH et al (2020) Final results from the phase I study expansion cohort of the selective FGFR inhibitor Debio 1,347 in patients with solid tumors harboring an FGFR gene fusion. J Clin Oncol 3603–3603
Valle JW, Bibeau K, Cho Y et al (2021) Longitudinal evaluation of quality of life (QoL) in patients (Pts) with FGFR2-driven cholangiocarcinoma (CCA) treated with pemigatinib. J Clin Oncol 39:276–276
Bekaii-Saab TS, Valle JW, Van Cutsem E et al (2020) FIGHT-302: Phase III study of first-line (1L) pemigatinib (PEM) versus gemcitabine (GEM) plus cisplatin (CIS) for cholangiocarcinoma (CCA) with FGFR2 fusions or rearrangements. J Clin Oncol 38: abstr TPS592
Javle MM, Borbath I, Clarke SJ et al (2019) Infigratinib versus gemcitabine plus cisplatin multicenter, open-label, randomized, phase 3 study in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: the PROOF trial. J Clin Oncol 37.15 suppl
Goyal L, Saha SK, Liu LY et al (2017) Polyclonal Secondary FGFR2 Mutations Drive Acquired Resistance to FGFR Inhibition in Patients with FGFR2 Fusion-Positive Cholangiocarcinoma. Cancer Discov 7:252–263
Goyal L, Shi L, Liu LY et al (2019) TAS-120 overcomes resistance to ATP-competitive FGFR inhibitors in patients with FGFR2 Fusion-Positive intrahepatic cholangiocarcinoma. Cancer Disc 9(8):1064–1079
Funding
This work was supported by the National Institute of Health (NIH) through a DP2 Award CA195764 (to MJB); National Cancer Institute (NCI) K12 award CA090628 (to MJB), SPORE Project Award 5P50CA210964-03 (MJB), Mayo Clinic Center for Individualized Medicine (CIM) Precision Cancer Therapeutics Program; and Mayo Clinic Cancer Center. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
Daniel Ahn has written the manuscript and created the tables, assisted in the design, and final approval of the manuscript. Pedro Uson Junior assisted in writing the manuscript, review, and final approval of the manuscript. Mitesh Borad has assisted in the design, writing, review, and final approval of the manuscript. Peter Masci, Heidi Kosiorek, Thorvardur R. Halfdanarson, Kabir Mody, Hani Babiker, Thomas DeLeon, Mohamad Bassam Sonbol, Gregory Gores, Rory Smoot, Tanios Bekaii-Saab, Amit Mahipal, Aaron Mansfield, Nguyen H Tran, Joleen M Hubbard assisted in the design, review, and final approval of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Mayo Clinic Institutional Review Board (IRB).
Consent to participate
All participants provided informed consent as outlined in the manuscript.
Consent for publication
All authors approved the final version of this manuscript.
Conflicts of interest
MJB has received grant to institution from Senhwa Pharmaceuticals, Adaptimmune, Agios Pharmaceuticals, Halozyme Pharmaceuticals, Five Prime Pharmaceuticals, Celgene Pharmaceuticals, EMD Merck Serono, Toray, Dicerna, Taiho Pharmaceuticals, Sun Biopharma, Isis Pharmaceuticals, Redhill Pharmaceuticals, Boston Biomed, Basilea, Incyte Pharmaceuticals, Mirna Pharmaceuticals, Medimmune, Bioline, Sillajen, ARIAD Pharmaceuticals, PUMA Pharmaceuticals, Novartis Pharmaceuticals, QED Pharmaceuticals, Pieris Pharmaceuticals, consultancy from ADC Therapeutics, Exelixis Pharmaceuticals, Inspyr Therapeutics, G1 Therapeutics, Immunovative Therapies, OncBioMune Pharmaceuticals, Western Oncolytics, Lynx Group, and travel support from Astra Zeneca.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ahn, D.H., Uson Junior, P.L.S., Masci, P. et al. A pilot study of Pan-FGFR inhibitor ponatinib in patients with FGFR-altered advanced cholangiocarcinoma. Invest New Drugs 40, 134–141 (2022). https://doi.org/10.1007/s10637-021-01170-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01170-x