Summary
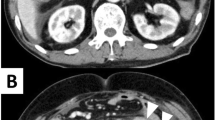
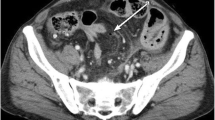
Gastrointestinal perforation related to mitogen-activated protein kinase kinase (MEK) inhibitors has been reported previously; however, there has been no case report of such a condition in patients with non-small cell lung cancer (NSCLC). Herein, we report a case of small intestinal perforation secondary to dabrafenib and trametinib administration, but not related to tumor regression. A 62-year-old man with non-small cell lung cancer harboring BRAF V600E mutation was treated with dabrafenib and trametinib. Four months after the initiation of treatment, a small intestinal perforation was diagnosed. Dabrafenib and trametinib rechallenge was performed after gastrointestinal perforation. The patient responded well to therapy and did not experience recurrence of gastrointestinal perforation. To the best of our knowledge, this is the first report of gastrointestinal perforation in a patient with NSCLC treated with a MEK inhibitor. The mechanism and risk factors of trametinib-induced perforation are currently unknown. Physicians should be aware of such severe gastrointestinal side effects of trametinib.


Similar content being viewed by others
Data availability
All related datas are presented in this paper, further inofrmation or datas are available on a reasonable request.
Abbreviations
- BRAF:
-
V-raf murine sarcoma viral oncogene homolog B1
- MEK:
-
Mitogen-activated protein kinase kinase
- CT:
-
Computed tomography
- NSCLC:
-
Non-small cell lung cancer
- PR:
-
Partial response
References
Cardarella S, Ogino A, Nishino M et al (2013) Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res 19:4532–4540. https://doi.org/10.1158/1078-0432.CCR-13-0657
Pratilas CA, Hanrahan AJ, Halilovic E et al (2008) Genetic predictors of MEK dependence in non–small cell lung cancer. Cancer Res 68:9375–9383. https://doi.org/10.1158/0008-5472.CAN-08-2223
Solit DB, Garraway LA, Pratilas CA et al (2006) BRAF mutation predicts sensitivity to MEK inhibition. Nature 439:358–362. https://doi.org/10.1038/nature04304
Planchard D, Besse B, Groen HJ et al (2016) Dabrafenib plus trametinib in patients with previously treated BRAFV600E-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 17:984–993. https://doi.org/10.1016/S1470-2045(16)30146-2
Planchard D, Smit EF, Groen HJ et al (2017) Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 18:1307–1316. https://doi.org/10.1016/S1470-2045(17)30679-4
Kalemkerian GP, Narula N, Kennedy EB et al (2018) Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of The College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology clinical practice guideline update. J Clin Oncol 36:911–919. https://doi.org/10.1200/JCO.2017.76.7293
Ettinger DS, Wood DE et al (2020) NCCN Guidelines Version 1. 2021 Non-Small Cell Lung Cancer 2020. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed 25 November 2020. National Comprehensive Cancer Network
Mourad N, Lourenço N, Delyon J et al (2019) Severe gastrointestinal toxicity of MEK inhibitors. Melanoma Res 29:556–559. https://doi.org/10.1097/CMR.0000000000000618
Kass SL, Linden AF, Jackson PG, De Brito PA, Atkins MB (2015) Bowel perforation associated with robust response to BRAF/MEK inhibitor therapy for BRAF-mutant melanoma: a case report. Melanoma Manag 2:115–120. https://doi.org/10.2217/mmt.15.10
Ascierto PA, Schadendorf D, Berking C et al (2013) MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol 14:249–256. https://doi.org/10.1016/S1470-2045(13)70024-X
Uppaluri R, Winkler AE, Lin T et al (2017) Biomarker and tumor responses of oral cavity squamous cell carcinoma to trametinib: a phase II neoadjuvant window-of-opportunity clinical trial. Clin Cancer Res 23:2186–2194. https://doi.org/10.1158/1078-0432.CCR-16-1469
Goldstraw P, Chansky K, Crowley J et al (2016) The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 11:39–51. https://doi.org/10.1016/j.jtho.2015.09.009
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Common terminology criteria for adverse events (CTCAE) Version 5.0. (2017) https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf Accessed 27 November 2017. U.S. Department of Health and Human Services
Acknowledgements
The authors would like to thank Keiko Sakuragawa and Kanako Masuta for their administrative assistance, and Motoko Mizumoto for operation of the case.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Yuri Shimada, Yuki Sato, Ryo Tachikawa and Shigeo Hara. The first draft of the manuscript was written by Yuri Shimada and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for publication
Informed consent was obtained from the patient.
Competing interests
Dr. Sato and Dr. Tomii have received lecture fees from Novartis Pharma K.K. (Tokyo, Japan). All remaining authors have no conflicts of interest to declare. We wish to confirm that there are no other known conflicts of interest associated with this publication. Further, there was no significant financial support for this work that could have influenced its outcome.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shimada, Y., Sato, Y., Tachikawa, R. et al. Gastrointestinal perforation following dabrafenib and trametinib administration in non-small cell lung carcinoma with BRAF V600E mutation: a case report and literature review. Invest New Drugs 39, 1702–1706 (2021). https://doi.org/10.1007/s10637-021-01135-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01135-0