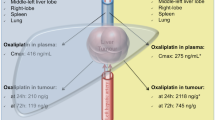
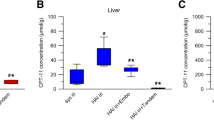
Summary
Background Tirapazamine’s (TPZ) tolerability after an intra-arterial (IA) injection remains unclear. We investigated TPZ’s safety and tolerability in rats by first injecting into the left hepatic artery and then performing a hepatic artery ligation, which recapitulates the transarterial embolization used clinically. Research design and methods: Forty-six rats in five groups were respectively injected with 0, 0.25, 0.50, 1.0, or more than 1.5 mL IA of TPZ (0.7 mg/mL) into the left hepatic artery and then subjected to hepatic artery ligation under laparotomy. Blood samples were collected four times daily up to day 15 after which the rats were euthanized and necropsied. The toxicity profile of IA injection of TPZ followed by hepatic artery ligation was then assessed. Results No significant changes to the rats’ body weight and serum total bilirubin were observed. Serum alanine aminotransferase (ALT) levels increased slightly but remained below 100 U/L one day after treatment for most rats. Three rats in Groups 3 and 4 exhibited an over two-fold transient elevation of ALT. All ALT recovered to the baseline at day 14. Liver tissues were collected on day 15 using H&E staining. One rat in Group 3 showed ischemic coagulative necrosis in its liver tissue. Other sporadic pathological changes not related to TPZ doses were observed in Groups 2, 3, 4, and 5. Conclusion TPZ by IA injection followed by embolization is tolerated up to 7 mg/kg. This finding supports the strategy of administering an IA injection of TPZ followed by trans-arterial embolization to the liver.





Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- ALT:
-
alanine aminotransferase
- HBx:
-
Hepatitis B virus X protein
- HCC:
-
Hepatocellular carcinoma
- IA:
-
Intra-arterial
- IV:
-
Intravenous
- MTD:
-
Maximum tolerated dose
- TPZ:
-
Tirapazamine
References
Brown JM (1993) SR 4233 (tirapazamine): a new anticancer drug exploiting hypoxia in solid tumours. Br J Cancer 67(6):1163–1170
Gandara DR, Lara PN Jr, Goldberg Z, Le QT, Mack PC, Lau DH, Gumerlock PH (2002) Tirapazamine: prototype for a novel class of therapeutic agents targeting tumor hypoxia. Semin Oncol 29(1 Suppl 4):102–109. https://doi.org/10.1053/sonc.2002.31531
Senan S, Rampling R, Graham MA, Wilson PJ, Robin H, Eckardt NA, Lawson N, McDonald AD, Roemeling RV, Workman P, Kaye SB (1997) Phase I and pharmacokinetic study of tirapazamine (SR 4233) administered every three weeks. Clin Cancer Res 3(1):31–38
Covans A, Blessing J, Bender D, Mannel R, Morgan M (2006) A phase II evaluation of tirapazamine plus cisplatin in the treatment of recurrent platinum-sensitive ovarian or primary peritoneal cancer: a gynecologic oncology group study. Gynecol Oncol. 100(3):586–590. https://doi.org/10.1016/j.ygyno.2005.09.032
Maluf FC, Leiser AL, Aghajanian C, Sabbatini P, Pezzulli S, Chi DS, Wolf JK, Levenback C, Loh E, Spriggs DR (2006) Phase II study of tirapazamine plus cisplatin in patients with advanced or recurrent cervical cancer. Int J Gynecol Cancer 16(3):1165–1171. https://doi.org/10.1111/j.1525-1438.2006.00454.x
Le QT, Taira AL, Budenz S, Jo Dorie M, Goffinet DR, Fee WE, Goode R, Bloch D, Koong A, Martin Brown J, Pinto HA (2006) Mature results from a randomized phase II trial of cisplatin plus 5-fluorouracil and radiotherapy with or without tirapazamine in patients with resectable stage IV head and neck squamous cell carcinomas. Cancer. 106(9):1940–1949. https://doi.org/10.1002/cncr.21785
Williamson SK, Crowley JJ, Lara PN Jr, McCoy J, Lau DH, Tucker RW, Mills GM, Gandara DR (2005) Phase III trial of paclitaxel plus carboplatin with or without tirapazamine in advanced non-small-cell lung cancer: southwest oncology group trial S0003. J Clin Oncol 23(36):9097–9104. https://doi.org/10.1200/JCO.2005.01.3771
Rischin D, Peters LJ, O’Sullivan B, Giralt J, Fisher R, Yuen K, Trotti A, Bernier J, Bourhis J, Ringash J, Henke M (2010) Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the trans-Tasman radiation oncology group. J Clin Oncol 28(18):2989–2995. https://doi.org/10.1200/JCO.2009.27.4449
DiSilvestro PA, Ali S, Craighead PS, Lucci JA, Lee YC, Cohn DE, Spirtos NM, Tewari KS, Muller C, Gajewski WH, Steinhoff MM (2014) Phase III randomized trial of weekly cisplatin and irradiation versus cisplatin and tirapazamine and irradiation in stages IB2, IIA, IIB, IIIB, and IVA cervical carcinoma limited to the pelvis: a gynecologic oncology group study. J Clin Oncol 32(5):458–464. https://doi.org/10.1200/JCO.2013.51.4265
Sonada A, Nitta N, Ohta S, Nagatani Y, Takahashi M, Marata K (2011) Enhanced antitumor effect of tirapazamine delivered intraperitoneally to VX2 liver tumor-bearing rabbits subjected to transarterial hepatic embolization. Cardiovasc Intervent Radiol 34(6):1272–1277. https://doi.org/10.1007/s00270-011-0156-4
Lin WH, Yeh SH, Yeh KH, Chen KW, Cheng YW, Su TH, Jao P, Ni LC, Chen PJ, Chen DS (2016) Hypoxia-activated cytotoxic agent tirapazamine enhances hepatic artery ligation-induced killing of liver tumor in HBx transgenic mice. Proc Natl Acad Sci U S A 113(42):11937–11942. https://doi.org/10.1073/pnas.1613466113
Govaert KM, Emmink BL, Nijkamp MW, Cheung ZJ, Steller EJA, Fatrai S, de Bruijn MT, Kranenburg O, Rinkes IHMB (2014) Hypoxia after liver surgery imposes an aggressive cancer stem cell phenotype on residual tumor cells. Ann Surg 259(4):750–759
Graham MA, Senan S, Robin H Jr, Eckhardt N, Lendrem D, Hincks J, Greenslade D, Rampling R, Kaye SB, Von Roemeling R, Workman P (1997) Pharmacokinetics of the hypoxic cell cytotoxic agent tirapazamine and its major bioreductive metabolites in mice and humans: retrospective analysis of a pharmacokinetically guided dose-escalation strategy in a phase I trial. Cancer Chemother Pharmacol 40(1):1–10
Acknowledgements
We thank Dr. Ray Lee for helpful discussion and suggestions, and thank Cheery Pharma Co. Ltd. for providing tirapazamine and funding for the study.
Funding
The work was supported by Cheery Pharma Co. Ltd.
Author information
Authors and Affiliations
Contributions
CHL and SMK conceived and designed research. SMK, HWG, and YJH conducted the experiments. SMK, HWG, and YJH analyzed data. CHL and SMK wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Consent for publication
Not applicable.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Figure 1
Changes of creatinine in the 5 groups (Normal range: Male: 0.43-0.68 mg/dL; Female: 0.5-0.7 mg/dL) (PNG 2911 kb)
Figure 2
Changes of glucose in the 5 groups (Normal range: Male: 148.8-231 mg/dL; Female: 141.6-209.8 mg/dL) (PNG 2718 kb)
Figure 3
Changes of potassium in the 5 groups (Normal range: Male: 4-5.2 mmol/L; Female: 3.6-5 mmol/L) (PNG 2983 kb)
Figure 4
Changes of sodium in the 5 groups (Normal range: Male: 139.2-147.8 mmol/L; Female: 139.4-146.8 mmol/L) (PNG 3457 kb)
Rights and permissions
About this article
Cite this article
Liu, CH., Kuo, SM., Gao, HW. et al. A rat toxicological study of intra-arterial injection of Tirapazamine, a hypoxia-activating Cancer therapeutic agent, followed by hepatic artery ligation. Invest New Drugs 39, 747–755 (2021). https://doi.org/10.1007/s10637-020-01057-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-01057-3