Summary
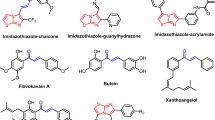
Two novel chemotherapeutic chalcones were synthesized and their structures were confirmed by different spectral tools. Theoretical studies such as molecular modeling were done to detect the mechanism of action of these compounds. In vitro cytotoxicity showed a strong effect against all tested cell lines (MCF7, A459, HepG2, and HCT116), and low toxic effect against normal human melanocytes (HFB4). The lung carcinoma cell line was chosen for further molecular studies. Real-time PCR demonstrated that the two compounds upregulated gene expression of (BAX, p53, casp-3, casp-8, casp-9) genes and decreased the expression of anti-apoptotic genes bcl2, CDK4, and MMP1. Flow-cytometry indicated that cell cycle arrest of A459 was induced at the G2/M phase and the apoptotic percentage increased significantly compared to the control sample. Cytochrome c oxidase and VEGF enzyme activity were detected by ELISA assay. SEM tool was used to follow the morphological changes that occurred on the cell surface, cell granulation, and average roughness of the cell surface. The change in the number and morphology of mitochondria, cell shrinkage, increase in the number of cytoplasmic organelles, membrane blebbing, chromatin condensation, and apoptotic bodies were observed using TEM. The obtained data suggested that new chalcones exerted their pathways on lung carcinoma through induction of two pathways of apoptosis.

Novel chalcones were prepared and confirmed by different spectral tools. Docking simulations were done to detect the mechanism of action. In vitro cytotoxicity indicated a strong effect against different cancer cell lines and low toxic effects against normal human melanocytes (HFB4). The lung carcinoma cell line was chosen for further molecular studies that include Real-time PCR, Flow-cytometry, Cytochrome c oxidase, and ELISA assay. SEM and TEM tool were used to follow the morphological changes occurred on the cell surface






Similar content being viewed by others
References
Elwan NM, Abdelhadi HA, Abdallah TA, Hassaneen HM (1996) Synthesis of [1,2,4]triazolo[3,4-a]isoquinolines and pyrrolo[2,1-a]isoquinolines using α-keto hydrazonoyl halides. Tetrahedron 52:3451–3456
Bekhit AA, Fahmy HTY (2003) Design and Synthesis of Some Substituted 1H-Pyrazolyl-oxazolidines or 1H-Pyrazolyl-thiazolidines as Anti-inflammatory-Antimicrobial Agents. Arch Pharm 336:111–118
Kalaria PN, Satasia SP, Raval DK (2014) Synthesis, characterization and pharmacological screening of some novel 5-imidazopyrazole incorporated polyhydroquinoline derivatives. Eur J Med Chem 78:207–216
Finn J, Mattia K, Morytko M, Ram S, Yang Y, Wu X, Mak E, Gallant P, Keith D (2003) Discovery of a potent and selective series of pyrazole bacterial methionyl-tRNA synthetase inhibitors. Bioorg Med Chem Lett 13:2231–2234
Tanitame A, Oyamada Y, Ofuji K, Fujimoto M, Suzuki K, Ueda T, Terauchi H, Kawasaki M, Nagai K, Wachi M, Yamagishi J-i (2004) Synthesis and antibacterial activity of novel and potent DNA gyrase inhibitors with azole ring. Bioorg Med Chem 12:5515–5524
Li Y, Zhang H-Q, Liu J, Yang X-P, Liu Z-J (2006) Stereoselective Synthesis and Antifungal Activities of (E)-α-(Methoxyimino)benzeneacetate Derivatives Containing 1,3,5-Substituted Pyrazole Ring. J Agric Food Chem 54:3636–3640
Zampieri D, Mamolo MG, Laurini E, Scialino G, Banfi E, Vio L (2008) Antifungal and antimycobacterial activity of 1-(3,5-diaryl-4,5-dihydro-1H-pyrazol-4-yl)-1H-imidazole derivatives. Bioorg Med Chem 16:4516–4522
Kocyigit-Kaymakcioglu B, Aker RG, Tezcan K, Sakalli E, Ketenci S, Oruç-Emre EE, Akin D, Gurbanova A, Terzioglu B, Onat F, Rollas S (2010) Anticonvulsant activity of 3,5-dimethylpyrazole derivatives in animal models. Med Chem Res 20:607–614
Kaushik D, Khan SA, Chawla G, Kumar S (2010) N’-[(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene] 2/4-substituted hydrazides: Synthesis and anticonvulsant activity. Eur J Med Chem 45:3943–3949
Palaska E, Aydin F, Uçar G, Erol D (2008) Synthesis and Monoamine Oxidase Inhibitory Activities of 1-Thiocarbamoyl-3,5-diphenyl-4,5-dihydro-1H-pyrazole Derivatives. Arch Pharm 341:209–215
Siddiqui N, Alam P, Ahsan W (2009) Design, Synthesis, andIn-VivoPharmacological Screening ofN,3-(Substituted Diphenyl)-5-phenyl-1H-pyrazoline-1-carbothioamide Derivatives. Arch Pharm 342:173–181
Bekhit AA, Hymete A, Asfaw H, Bekhit AE-DA (2012) Synthesis and Biological Evaluation of Some Pyrazole Derivatives as Anti-Malarial Agents. Arch Pharm 345:147–154
Abadi AH, Eissa AAH, Hassan GS (2003) Synthesis of Novel 1,3,4-Trisubstituted Pyrazole Derivatives and Their Evaluation as Antitumor and Antiangiogenic Agents. Chem Pharm Bull 51:838–844
Baraldi PG, Beria I, Cozzi P, Geroni C, Espinosa A, Gallo MA, Entrena A, Bingham JP, Hartley JA, Romagnoli R (2004) Cinnamoyl nitrogen mustard derivatives of pyrazole analogues of tallimustine modified at the amidino moiety: design, synthesis, molecular modeling and antitumor activity studies. Bioorg Med Chem 12:3911–3921
Cheung K-MJ, Matthews TP, James K, Rowlands MG, Boxall KJ, Sharp SY, Maloney A, Roe SM, Prodromou C, Pearl LH, Aherne GW, McDonald E, Workman P (2005) The identification, synthesis, protein crystal structure and in vitro biochemical evaluation of a new 3,4-diarylpyrazole class of Hsp90 inhibitors. Bioorg Med Chem Lett 15:3338–3343
Bouabdallah I, M’Barek LA, Zyad A, Ramdani A, Zidane I, Melhaoui A (2006) Anticancer effect of three pyrazole derivatives. Nat Prod Res 20:1024–1030
Ohki H, Hirotani K, Naito H, Ohsuki S, Minami M, Ejima A, Koiso Y, Hashimoto Y (2002) Synthesis and mechanism of action of novel pyrimidinyl pyrazole derivatives possessing antiproliferative activity. Bioorg Med Chem Lett 12:3191–3193
Mulder R, Wellinga K, van Daalen JJ (1975) A new class of insecticides. Naturwissenschaften 62:531–532
Hsieh H-K, Tsao L-T, Wang J-P, Lin C-N (2000) Synthesis and Anti-inflammatory Effect of Chalcones. J Pharm Pharmacol 52:163–171
Bandgar BP, Gawande SS, Bodade RG, Gawande NM, Khobragade CN (2009) Synthesis and biological evaluation of a novel series of pyrazole chalcones as anti-inflammatory, antioxidant and antimicrobial agents. Bioorg Med Chem 17:8168–8173
Bekhit AA, Abdel-Aziem T (2004) Design, synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory-antimicrobial agents. Bioorg Med Chem 12:1935–1945
Lin C-N, Hsieh H-K, Ko H-H, Hsu M-F, Lin H-C, Chang Y-L, Chung M-I, Kang J-J, Wang J-P, Teng C-M (2001) Chalcones as potent antiplatelet agents and calcium channel blockers. Drug Dev Res 53:9–14
Heidari MR, Foroumadi A, Amirabadi A, Samzadeh-Kermani A, Azimzadeh BS, Eskandarizadeh A (2009) Evaluation of anti-inflammatory and analgesic activity of a novel rigid 3, 4-dihydroxy chalcone in mice. Ann N Y Acad Sci 1171:399–406
Li R, Kenyon GL, Cohen FE, Chen X, Gong B, Dominguez JN, Davidson E, Kurzban G, Miller RE, Nuzum EO, Rosenthal PJ, McKerrow JH (1995) In Vitro Antimalarial Activity of Chalcones and Their Derivatives. J Med Chem 38:5031–5037
Shenvi S, Kumar K, Hatti KS, Rijesh K, Diwakar L, Reddy GC (2013) Synthesis, anticancer and antioxidant activities of 2,4,5-trimethoxy chalcones and analogues from asaronaldehyde: Structure–activity relationship. Eur J Med Chem 62:435–442
Onyilagha JC, Malhotra B, Elder M, French CJ, Towers GHN (1997) Comparative studies of inhibitory activities of chalcones on tomato ringspot virus (ToRSV). Can J Plant Path 19:133–137
Asiri AM, Khan SA (2011) Synthesis and Anti-Bacterial Activities of a Bis-Chalcone Derived from Thiophene and Its Bis-Cyclized Products. Molecules 16:523–531
Mohamed MF, Mohamed MS, Shouman SA, Fathi MM, Abdelhamid IA (2012) Synthesis and Biological Evaluation of a Novel Series of Chalcones Incorporated Pyrazole Moiety as Anticancer and Antimicrobial Agents. Appl Biochem Biotechnol 168:1153–1162
Tantawy MA, Sroor FM, Mohamed MF, El-Naggar ME, Saleh FM, Hassaneen HM, Abdelhamid IA (2020) Molecular Docking Study, Cytotoxicity, Cell Cycle Arrest and Apoptotic Induction of Novel Chalcones Incorporating Thiadiazolyl Isoquinoline in Cervical Cancer. Anticancer Agents Med Chem 20:70–84
Sashidhara KV, Kumar A, Kumar M, Sarkar J, Sinha S (2010) Synthesis and in vitro evaluation of novel coumarin–chalcone hybrids as potential anticancer agents. Bioorg Med Chem Lett 20:7205–7211
Ibrahim NS, Mohamed MF, Elwahy AHM, Abdelhamid IA (2018) Biological Activities and Docking Studies on Novel Bis 1,4-DHPS Linked to Arene Core via Ether or Ester Linkage. Lett Drug Des Discovery 15:1036–1045
Abdella AM, Mohamed MF, Mohamed AF, Elwahy AHM, Abdelhamid IA (2018) Novel bis(dihydropyrano[3,2-c]chromenes): Synthesis, Antiproliferative Effect and Molecular Docking Simulation. J Heterocycl Chem 55:498–507
Sroor FM, Abbas SY, Basyouni WM, El-Bayouki KAM, El-Mansy MF, Aly HF, Ali SA, Arafa AF, Haroun AA (2019) Synthesis, structural characterization and in vivo anti-diabetic evaluation of some new sulfonylurea derivatives in normal and silicate coated nanoparticle forms as anti-hyperglycemic agents. Bioorg Chem 92:103290
Sroor FM, Abdelmoniem AM, Abdelhamid IA (2019) Facile Synthesis, Structural Activity Relationship, Molecular Modeling and In Vitro Biological Evaluation of New Urea Derivatives with Incorporated Isoxazole and Thiazole Moieties as Anticancer Agents. ChemistrySelect 4:10113–10121
Sroor FM, Basyouni WM, Tohamy WM, Abdelhafez TH, El-awady MK (2019) Novel pyrrolo[2,3-d]pyrimidine derivatives: Design, synthesis, structure elucidation and in vitro anti-BVDV activity. Tetrahedron 75:130749
Sroor FM, Khatab TK, Basyouni WM, El-Bayouki KAM (2019) Synthesis and molecular docking studies of some new thiosemicarbazone derivatives as HCV polymeraseinhibitors. Synth Commun 49:1444–1456
Mohamed MF, Samir N, Ali A, Ahmed N, Ali Y, Aref S, Hossam O, Mohamed MS, Abdelmoniem AM, Abdelhamid IA (2017) Apoptotic induction mediated p53 mechanism and Caspase-3 activity by novel promising cyanoacrylamide derivatives in breast carcinoma. Bioorg Chem 73:43–52
Mohamed MF, Hassaneen HM, Abdelhamid IA (2018) Cytotoxicity, molecular modeling, cell cycle arrest, and apoptotic induction induced by novel tetrahydro-[1,2,4]triazolo[3,4-a]isoquinoline chalcones. Eur J Med Chem 143:532–541
Ghozlan SAS, Mohamed MF, Ahmed AG, Shouman SA, Attia YM, Abdelhamid IA (2015) Cytotoxic and Antimicrobial Evaluations of Novel Apoptotic and Anti-Angiogenic Spiro Cyclic 2-Oxindole Derivatives of 2-Amino-tetrahydroquinolin-5-one. Arch Pharm 348:113–124
Mohamed MF, Elhakim HKA, Saddiq AA, Abdelhamid IA (2020) A novel inhibitor, 2-cyano-3-(1-phenyl-3-(thiophen-2-yl)-pyrazol-4-yl)acrylamide linked to sulphamethoxazole, blocks anti-apoptotic proteins via molecular docking and strongly induced apoptosis of HCT116 Cell line by different molecular tools. Arab J Chem 13(7): 5978–5995
Khatab TK, El-Bayouki KAM, Basyouni WM, Sroor FMA (2013) An Efficient Synthesis of Biopertinent Dihydropyrimidine (thi) one Derivatives via Threecomponent One-pot Synthesis Catalyzed by Tetrachlorosilane. Egypt J Chem 56:291–305
Abdelmoniem AM, Mohamed MF, Abdelmoniem DM, Ghozlan SAS, Abdelhamid IA (2019) Recent Synthetic Approaches and Biological Evaluations of Amino Hexahydroquinolines and Their Spirocyclic Structures. Anticancer Agents Med Chem 19:875–915
Sroor FM, Aboelenin MM, Mahrous KF, Mahmoud K, Elwahy AHM, Abdelhamid IA (2020) Novel 2-cyanoacrylamido‐4,5,6,7‐tetrahydrobenzo[b]thiophene derivatives as potent anticancer agents. Arch Pharm. https://doi.org/10.1002/ardp.202000069
Abdelhamid IA, Abdelmoniem AM, Sroor FM, Ramadan MA, Ghozlan SAS (2020) Hantzsch-Like One-Pot Three-Component Synthesis of Heptaazadicyclopenta[a,j]anthracenes: A New Ring System. Synlett 31:895–898
Hassaneen HM, Hassaneen HME, Mohammed YS, Pagni RM (2011) Synthesis, Reactions and Antibacterial Activity of 3-Acetyl[1,2,4]triazolo[3,4-a]isoquinoline Derivatives using Chitosan as Heterogeneous Catalyst under Microwave Irradiation. Zeitschrift für Naturforschung B 66:299–310
Wang Y, Hedblom A, Koerner SK, Li M, Jernigan FE, Wegiel B, Sun L (2016) Novel synthetic chalcones induce apoptosis in the A549 non-small cell lung cancer cells harboring a KRAS mutation. Bioorg Med Chem Lett 26:5703–5706
Pedrini FS, Chiaradia LD, Licínio MA, De Moraes ACR, Curta JC, Costa A, Mascarello A, Creczinsky-Pasa TB, Nunes RJ, Yunes RA, Santos-Silva MC (2010) Induction of apoptosis and cell cycle arrest in L-1210 murine lymphoblastic leukaemia cells by (2E)-3-(2-naphthyl)-1-(3′-methoxy-4′-hydroxy-phenyl)-2-propen-1-one. J Pharm Pharmacol 62:1128–1136
Rozmer Z, Berki T, Perjési P (2006) Different effects of two cyclic chalcone analogues on cell cycle of Jurkat T cells. Toxicol In Vitro 20:1354–1362
Rao YK, Fang S-H, Tzeng Y-M (2004) Differential effects of synthesized 2′-oxygenated chalcone derivatives: modulation of human cell cycle phase distribution. Bioorg Med Chem 12:2679–2686. https://doi.org/10.1016/j.bmc.2004.03.014
Brunhofer-Bolzer G, Le T, Dyckmanns N, Knaus HA, Pausz C, Freund P, Jäger U, Erker T, Vanura K (2015) SAR-Guided Development and Characterization of a Potent Antitumor Compound toward B-Cell Neoplasms with No Detectable Cytotoxicity toward Healthy Cells. J Med Chem 58:1244–1253
Alonso J-R, Cardellach F, Lopez S, Casademont J, Miro O (2003) Carbon Monoxide Specifically Inhibits Cytochrome C Oxidase of Human Mitochondrial Respiratory Chain. Pharmacol Toxicol 93:142–146
Nicholls P, Marshall Doug C, Cooper Chris E, Wilson Mike T (2013) Sulfide inhibition of and metabolism by cytochrome c oxidase. Biochem Soc Trans 41:1312–1316
Ali AG, Mohamed MF, Abdelhamid AO, Mohamed MS (2017) A novel adamantane thiadiazole derivative induces mitochondria-mediated apoptosis in lung carcinoma cell line. Bioorg Med Chem 25:241–253
Ishiwata T, Hasegawa F, Michishita M, Sasaki N, Ishikawa N, Takubo K, Matsuda Y, Arai T, Aida J (2018) Electron microscopic analysis of different cell types in human pancreatic cancer spheres. Oncol Lett 15:2485–2490
Funding
This work was supported by the personal fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 364 KB)
Rights and permissions
About this article
Cite this article
Mohamed, M.F., Sroor, F.M., Ibrahim, N.S. et al. Novel [l,2,4]triazolo[3,4-a]isoquinoline chalcones as new chemotherapeutic agents: Block IAP tyrosine kinase domain and induce both intrinsic and extrinsic pathways of apoptosis. Invest New Drugs 39, 98–110 (2021). https://doi.org/10.1007/s10637-020-00987-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-00987-2