Summary
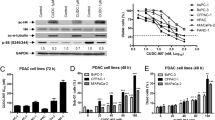
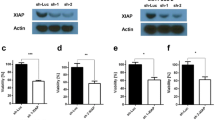
Inhibitor of apoptosis proteins (IAPs) are overexpressed in the majority of cancers and prevent apoptosis by inhibiting caspases. IAPs have therefore attracted considerable attention as potential targets for anticancer therapy. Here, we demonstrated that HM90822 (abbreviated HM822; a new synthetic IAP antagonist) induced apoptotic cell death via proteasome-dependent degradation of BIR2/3 domain-containing IAPs in human pancreatic cancer cells. HM822 inhibited the expression of XIAP and cIAP1/2 proteins in Panc-1 and BxPC-3 cells, which are sensitive to HM822. HM822 also induced IAP ubiquitination and promoted proteasome-dependent IAP degradation. However, cells expressing phospho-XIAP (Ser87) and AKT exhibited resistance to HM822. In other words, the overexpression of AKT-CA (constitutive active form for AKT) or AKT-WT induced resistance to HM822. In addition, in Panc-1 xenograft and orthotopic mouse models, we revealed that tumor growth was suppressed by the administration of HM822. Taken together, these results suggest that HM822 induces apoptosis through ubiquitin/proteasome-dependent degradation of BIR3 domain-containing IAPs. These findings suggest that phospho-XIAP and phospho-AKT may be used as biomarkers for predicting the efficacy of HM822 in pancreatic cancer patients.





Similar content being viewed by others
Abbreviations
- IAP:
-
inhibitor of apoptosis proteins
- HM822:
-
HM90822
References
Sellam F, Harir N, Khaled MB, Mrabent NM, Salah R, Benchouk A, Diaf M (2015) Delayed diagnosis of pancreatic cancer reported as more common in a population of North African young adults. J Gastrointest Oncol 6(5):505–510. https://doi.org/10.3978/j.issn.2078-6891.2015.051
Long J, Zhang Y, Yu X, Yang J, LeBrun DG, Chen C, Yao Q, Li M (2011) Overcoming drug resistance in pancreatic cancer. Expert Opin Ther Targets 15(7):817–828. https://doi.org/10.1517/14728222.2011.566216
Ziegler DS, Kung AL (2008) Therapeutic targeting of apoptosis pathways in cancer. Curr Opin Oncol 20(1):97–103. https://doi.org/10.1097/CCO.0b013e3282f310f6
Nachmias B, Ashhab Y, Ben-Yehuda D (2004) The inhibitor of apoptosis protein family (IAPs): an emerging therapeutic target in cancer. Semin Cancer Biol 14(4):231–243. https://doi.org/10.1016/j.semcancer.2004.04.002
Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD (2000) Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science 288(5467):874–877
Verhagen AM, Vaux DL (2002) Cell death regulation by the mammalian IAP antagonist Diablo/Smac. Apoptosis 7(2):163–166
Du C, Fang M, Li Y, Li L, Wang X (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102(1):33–42
Morizane Y, Honda R, Fukami K, Yasuda H (2005) X-linked inhibitor of apoptosis functions as ubiquitin ligase toward mature caspase-9 and cytosolic Smac/DIABLO. J Biochem 137(2):125–132
Esposito I, Kleeff J, Abiatari I, Shi X, Giese N, Bergmann F, Roth W, Friess H, Schirmacher P (2007) Overexpression of cellular inhibitor of apoptosis protein 2 is an early event in the progression of pancreatic cancer. J Clin Pathol 60(8):885–895. https://doi.org/10.1136/jcp.2006.038257
Che X, Yang D, Zong H, Wang J, Li X, Chen F, Chen X, Song X (2012) Nuclear cIAP1 overexpression is a tumor stage- and grade-independent predictor of poor prognosis in human bladder cancer patients. Urol Oncol 30(4):450–456. https://doi.org/10.1016/j.urolonc.2010.12.016
Moon JH, Shin JS, Hong SW, Jung SA, Hwang IY, Kim JH, Choi EK, Ha SH, Kim JS, Kim KM, Hong DW, Kim D, Kim YS, Kim JE, Kim KP, Hong YS, Lee JS, Hattersley M, Jin DH, Kim TW (2015) A novel small-molecule IAP antagonist, AZD5582, draws Mcl-1 down-regulation for induction of apoptosis through targeting of cIAP1 and XIAP in human pancreatic cancer. Oncotarget 6(29):26895–26908. https://doi.org/10.18632/oncotarget.4822
Lopes RB, Gangeswaran R, McNeish IA, Wang Y, Lemoine NR (2007) Expression of the IAP protein family is dysregulated in pancreatic cancer cells and is important for resistance to chemotherapy. Int J Cancer 120(11):2344–2352. https://doi.org/10.1002/ijc.22554
Yang QH, Du C (2004) Smac/DIABLO selectively reduces the levels of c-IAP1 and c-IAP2 but not that of XIAP and livin in HeLa cells. J Biol Chem 279(17):16963–16970. https://doi.org/10.1074/jbc.M401253200
Weissman AM (2001) Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol 2(3):169–178. https://doi.org/10.1038/35056563
Fu J, Jin Y, Arend LJ (2003) Smac3, a novel Smac/DIABLO splicing variant, attenuates the stability and apoptosis-inhibiting activity of X-linked inhibitor of apoptosis protein. J Biol Chem 278(52):52660–52672. https://doi.org/10.1074/jbc.M308036200
Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, Tsang BK, Cheng JQ (2004) Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP). J Biol Chem 279(7):5405–5412. https://doi.org/10.1074/jbc.M312044200
Acknowledgements
The authors would like to thank Dr. Colin S. Duckett of the University of Michigan Medical School for providing XIAP cDNAs.
Funding
This study was supported by grants from the Korea Health 21 R&D Project, Ministry of Health, Welfare and Family Affairs, Republic of Korea (A062254), and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (NRF-2017R1A2B4012721).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
Animal experiments were approved by the Laboratory Animal Research Committee of Asan Institute for Life Science, and all study procedures followed internationally accepted principles and the guidelines of care and use.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 350 kb)
Rights and permissions
About this article
Cite this article
Hong, SW., Shin, JS., Moon, JH. et al. Chemosensitivity to HM90822, a novel synthetic IAP antagonist, is determined by p-AKT-inducible XIAP phosphorylation in human pancreatic cancer cells. Invest New Drugs 38, 1696–1706 (2020). https://doi.org/10.1007/s10637-020-00956-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-00956-9