Summary
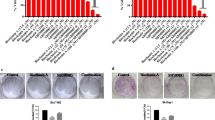
Hepatocellular carcinoma (HCC) is the most prevalent type of tumor among primary liver tumors and is the second highest cause of cancer-related deaths worldwide. Current therapies are controversial, and more research is needed to identify effective treatments. A new synthetic compound, potassium 5-cyano-4-methyl-6-oxo-1,6-dihydropyridine-2-olate (CPBMF65), is a potent inhibitor of the human uridine phosphorylase-1 (hUP1) enzyme, which controls the cell concentration of uridine (Urd). Urd is a natural pyrimidine nucleoside involved in cellular processes, such as RNA synthesis. In addition, it is considered a promising biochemical modulator, as it may reduce the toxicity caused by chemotherapeutics without impairing its anti-tumor activity. Thus, the objective of this study is to evaluate the effects of CPBMF65 on the proliferation of the human hepatocellular carcinoma cell line (HepG2). Cell proliferation, cytotoxicity, apoptosis, senescence, autophagy, intracellular Urd levels, cell cycle arrest, and drug resistance were analyzed. Results demonstrate that, after incubation with CPBMF65, HepG2 cell proliferation decreased, mainly through cell cycle arrest and senescence, increasing the levels of intracellular Urd and maintaining cell proliferation reduced during chronic treatment. In conclusion, results show, for the first time, the ability of a hUP1 inhibitor (CPBMF65) to reduce HepG2 cell proliferation through cell cycle arrest and senescence.






Similar content being viewed by others
References
Yang JD, Hainaut P, Gores GJ et al (2019) A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. https://doi.org/10.1038/s41575-019-0186-y
Sachdeva M (2015) Immunology of hepatocellular carcinoma. World J Hepatol 7:2080. https://doi.org/10.4254/wjh.v7.i17.2080
Attwa MH (2015) Guide for diagnosis and treatment of hepatocellular carcinoma. World J Hepatol 7:1632. doi:https://doi.org/10.4254/wjh.v7.i12.1632
Byam J, Renz J, Millis JM (2013) Liver transplantation for hepatocellular carcinoma. Hepatobiliary Surg Nutr 2:22–30. https://doi.org/10.3978/j.issn.2304-3881.2012.11.03
Sapisochin G, Bruix J (2017) Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol 14:203–217. https://doi.org/10.1038/nrgastro.2016.193
Renck D, MacHado P, Souto AA et al (2013) Design of novel potent inhibitors of human uridine phosphorylase-1: Synthesis, inhibition studies, thermodynamics, and in vitro influence on 5-fluorouracil cytotoxicity. J Med Chem. https://doi.org/10.1021/jm401389u
Renck D, Santos AA, Machado P et al (2014) Human uridine phosphorylase-1 inhibitors: A new approach to ameliorate 5-fluorouracil-induced intestinal mucositis. Invest New Drugs. https://doi.org/10.1007/s10637-014-0135-0
Pizzorno G, Cao D, Leffert JJ et al (2002) Homeostatic control of uridine and the role of uridine phosphorylase: a biological and clinical update. Biochim Biophys Acta Mol Basis Dis 1587:133–144. https://doi.org/10.1016/S0925-4439(02)00076-5
van Groeningen CJ, Peters GJ, Pinedo HM (1992) Modulation of fluorouracil toxicity with uridine. Semin Oncol. https://doi.org/10.5555/uri:pii:009377549290077E
Leyva A, van Groeningen CJ, Kraal I et al (1984) Phase I and pharmacokinetic studies of high-dose uridine intended for rescue from 5-fluorouracil toxicity. Cancer Res 44:5928–5933
Haute GV, Caberlon E, Squizani E et al (2015) Gallic acid reduces the effect of LPS on apoptosis and inhibits the formation of neutrophil extracellular traps. Toxicol Vitr. https://doi.org/10.1016/j.tiv.2015.10.005
Strober W (2015) Trypan Blue Exclusion Test of Cell Viability. In: Curr. Protoc. Immunol. Wiley, Hoboken, p A3.B.1-A3.B.3
Krause GC, Lima KG, Levorse V et al (2019) Exenatide induces autophagy and prevents the cell regrowth in HEPG2 cells. EXCLI J. https://doi.org/10.17179/excli2019-1415
Kim TM, Shin SK, Kim TW et al (2012) Elm tree bark extract inhibits HepG2 hepatic cancer cell growth via pro-apoptotic activity. J Vet Sci. https://doi.org/10.4142/jvs.2012.13.1.7
Filippi-Chiela EC, Oliveira MM, Jurkovski B et al (2012) Nuclear morphometric analysis (NMA): Screening of senescence, apoptosis and nuclear irregularities. PLoS One. https://doi.org/10.1371/journal.pone.0042522
ZDANOV S (2006) Establishment of H2O2-induced premature senescence in human fibroblasts concomitant with increased cellular production of H2O2. Ann N Y Acad Sci 1067:210–216. https://doi.org/10.1196/annals.1354.025
Lima KG, Krause GC, da Silva EFG et al (2018) Octyl gallate reduces ATP levels and Ki67 expression leading HepG2 cells to cell cycle arrest and mitochondria-mediated apoptosis. Toxicol Vitr. https://doi.org/10.1016/j.tiv.2017.12.017
Krause GC, Lima KG, Dias HB et al (2017) Liraglutide, a glucagon-like peptide-1 analog, induce autophagy and senescence in HepG2 cells. Eur J Pharmacol. https://doi.org/10.1016/j.ejphar.2017.05.015
Khemlina G, Ikeda S, Kurzrock R (2017) The biology of Hepatocellular carcinoma: Implications for genomic and immune therapies. Mol Cancer. https://doi.org/10.1186/s12943-017-0712-x
Donato MT, Tolosa L, Gómez-Lechón MJ (2015) Culture and functional characterization of human hepatoma HepG2 cells. Protoc Vitr Hepatocyte Res. https://doi.org/10.1007/978-1-4939-2074-7_5
Salminen A, Kaarniranta K, Kauppinen A (2013) Beclin 1 interactome controls the crosstalk between apoptosis, autophagy and inflammasome activation: Impact on the aging process. Ageing Res Rev 12:520–534. https://doi.org/10.1016/j.arr.2012.11.004
Pattingre S, Tassa A, Qu X et al (2005) Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. https://doi.org/10.1016/j.cell.2005.07.002
Da Silva EFG, Le Catyana Krause G, Lima KG et al (2016) Rapamycin and fructose-1,6-bisphosphate reduce the HEPG2 cell proliferation via increase of free radicals and apoptosis. Oncol Rep. https://doi.org/10.3892/or.2016.5111
Suzuki K, Matsubara H (2011) Recent advances in p53 research and cancer treatment. J Biomed Biotechnol 2011:1–7. https://doi.org/10.1155/2011/978312
Huang R, Xue R, Qu D et al (2017) Prp19 arrests cell cycle via Cdc5L in hepatocellular carcinoma cells. Int J Mol Sci. https://doi.org/10.3390/ijms18040778
Schmidt A, Durgan J, Magalhaes A, Hall A (2007) Rho GTPases regulate PRK2/PKN2 to control entry into mitosis and exit from cytokinesis. EMBO J 26:1624–1636. https://doi.org/10.1038/sj.emboj.7601637
Otto T, Sicinski P (2017) Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer 17:93–115. https://doi.org/10.1038/nrc.2016.138
Rundle S, Bradbury A, Drew Y, Curtin N (2017) Targeting the ATR-CHK1 Axis in cancer therapy. Cancers (Basel) 9:41. https://doi.org/10.3390/cancers9050041
Matheson CJ, Backos DS, Reigan P (2016) Targeting WEE1 Kinase in Cancer. Trends Pharmacol Sci 37:872–881. https://doi.org/10.1016/j.tips.2016.06.006
Collado M, Blasco MA, Serrano M (2007) Cellular senescence in cancer and aging. Cell 130:223–233. https://doi.org/10.1016/j.cell.2007.07.003
Luo Y, Leverson JD (2005) New opportunities in chemosensitization and radiosensitization: modulating the DNA-damage response. Expert Rev Anticancer Ther 5:333–342. https://doi.org/10.1586/14737140.5.2.333
Mills CC, Kolb E, Sampson VB (2018) Development of chemotherapy with cell-cycle inhibitors for adult and pediatric cancer therapy. Cancer Res 78:320–325. https://doi.org/10.1158/0008-5472.CAN-17-2782
Lee Y-H, Chen Y-Y, Yeh Y-L et al (2019) Stilbene compounds inhibit tumor growth by the induction of cellular senescence and the inhibition of telomerase activity. Int J Mol Sci 20:2716. https://doi.org/10.3390/ijms20112716
Sampson VB, Vetter NS, Zhang W et al (2016) Integrating mechanisms of response and resistance against the tubulin binding agent Eribulin in preclinical models of osteosarcoma. Oncotarget. https://doi.org/10.18632/oncotarget.13358
Cairns RA, Harris IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11:85–95. https://doi.org/10.1038/nrc2981
Squadrito F, Bitto A, Irrera N et al (2017) Pharmacological Activity and Clinical Use of PDRN. Front Pharmacol. https://doi.org/10.3389/fphar.2017.00224
Funding
This study was financed, in part, by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brasil. Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
EFGS, KGL, GCK, GVH, LP, AVC, RBG, BSB, HBD, CL, MCRG, BPC, GLA, LAB, MVFD, PM, and JRO declare no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals, performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva, E.F.G., Lima, K.G., Krause, G.C. et al. CPBMF65, a synthetic human uridine phosphorylase-1 inhibitor, reduces HepG2 cell proliferation through cell cycle arrest and senescence. Invest New Drugs 38, 1653–1663 (2020). https://doi.org/10.1007/s10637-020-00941-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-00941-2