Summary
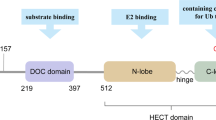
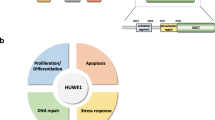
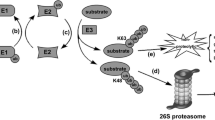
E3 ligases are a class of critical enzymes that can catalyse the transfer of ubiquitin (Ub) from an E2 enzyme to the substrate and are essential to cellular processes. The E3 ligase HUWE1 (also known as ARF-BP1, HECTH9, HSPC272, Ib772, LASU1, MULE, URE-B1, UREB1, and HECT, UBA and WWE domain-containing E3 ubiquitin protein ligase 1) is encoded by the huwe1 gene. HUWE1 is a key regulator of the DNA damage response, transcription, autophagy, apoptosis and metabolism in a variety of cancers. Due to its pivotal role in conferring substrate specificity, HUWE1 has attracted enormous attention as a promising anticancer drug target. In this review, we indicate the specific molecular structure of HUWE1 and its role in various cellular signalling pathways and highlight new insights into HUWE1 in cancer. Finally, we discuss outstanding questions regarding HUWE1 in oncology and highlight its limitations in drug development and clinical guidance to better define the role of HUWE1 in multiple cancers.





Similar content being viewed by others
References
Li Y, Xie P, Lu L, Wang J, Diao L, Liu Z, Guo F, He Y, Liu Y, Huang Q, Liang H, Li D, He F (2017) An integrated bioinformatics platform for investigating the human E3 ubiquitin ligase-substrate interaction network. Nat Commun 8(1):347. https://doi.org/10.1038/s41467-017-00299-9
Chen YJ, Wu H, Shen XZ (2016) The ubiquitin-proteasome system and its potential application in hepatocellular carcinoma therapy. Cancer Lett 379(2):245–252. https://doi.org/10.1016/j.canlet.2015.06.023
Glickman MH, Ciechanover A (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82(2):373–428. https://doi.org/10.1152/physrev.00027.2001
Jackl M, Stollmaier C, Strohaker T, Hyz K, Maspero E, Polo S, Wiesner S (2018) Beta-sheet augmentation is a conserved mechanism of priming HECT E3 ligases for ubiquitin ligation. J Mol Biol 430(18 Pt B):3218–3233. https://doi.org/10.1016/j.jmb.2018.06.044
Xu P, Duong DM, Seyfried NT, Cheng D, Xie Y, Robert J, Rush J, Hochstrasser M, Finley D, Peng J (2009) Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell 137(1):133–145. https://doi.org/10.1016/j.cell.2009.01.041
Varshavsky A (2017) The ubiquitin system, autophagy, and regulated protein degradation. Annu Rev Biochem 86:123–128. https://doi.org/10.1146/annurev-biochem-061516-044859
Nakayama KI, Nakayama K (2006) Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer 6(5):369–381. https://doi.org/10.1038/nrc1881
Schwechheimer C (2018) NEDD8-its role in the regulation of Cullin-RING ligases. Curr Opin Plant Biol 45(Pt A):112–119. https://doi.org/10.1016/j.pbi.2018.05.017
Ponts N, Saraf A, Chung DW, Harris A, Prudhomme J, Washburn MP, Florens L, Le Roch KG (2011) Unraveling the ubiquitome of the human malaria parasite. J Biol Chem 286(46):40320–40330. https://doi.org/10.1074/jbc.M111.238790
Martin P, Ruan J, Furman R, Rutherford S, Allan J, Chen Z, Huang X, DiLiberto M, Chen-Kiang S, Leonard JP (2019) A phase I trial of palbociclib plus bortezomib in previously treated mantle cell lymphoma. Leuk Lymphoma:1–5. https://doi.org/10.1080/10428194.2019.1612062
Ando M, Hoyos V, Yagyu S, Tao W, Ramos CA, Dotti G, Brenner MK, Bouchier-Hayes L (2014) Bortezomib sensitizes non-small cell lung cancer to mesenchymal stromal cell-delivered inducible caspase-9-mediated cytotoxicity. Cancer Gene Ther 21(11):472–482. https://doi.org/10.1038/cgt.2014.53
Singha B, Gatla HR, Manna S, Chang TP, Sanacora S, Poltoratsky V, Vancura A, Vancurova I (2014) Proteasome inhibition increases recruitment of IkappaB kinase beta (IKKbeta), S536P-p65, and transcription factor EGR1 to interleukin-8 (IL-8) promoter, resulting in increased IL-8 production in ovarian cancer cells. J Biol Chem 289(5):2687–2700. https://doi.org/10.1074/jbc.M113.502641
Gong L, Yang B, Xu M, Cheng B, Tang X, Zheng P, Jing Y, Wu GJ (2014) Bortezomib-induced apoptosis in cultured pancreatic cancer cells is associated with ceramide production. Cancer Chemother Pharmacol 73(1):69–77. https://doi.org/10.1007/s00280-013-2318-3
Li X, Pham V, Tippin M, Fu D, Rendon R, Song L, Uchio E, Hoang BH, Zi X (2019) Flavokawain B targets protein neddylation for enhancing the anti-prostate cancer effect of Bortezomib via Skp2 degradation. Cell Comm Signal: CCS 17(1):25. https://doi.org/10.1186/s12964-019-0338-2
Chauhan D, Singh A, Brahmandam M, Podar K, Hideshima T, Richardson P, Munshi N, Palladino MA, Anderson KC (2008) Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma. Blood 111(3):1654–1664. https://doi.org/10.1182/blood-2007-08-105601
Chen D, Kon N, Li M, Zhang W, Qin J, Gu W (2005) ARF-BP1/Mule is a critical mediator of the ARF tumor suppressor. Cell 121(7):1071–1083. https://doi.org/10.1016/j.cell.2005.03.037
Choe KN, Nicolae CM, Constantin D, Imamura Kawasawa Y, Delgado-Diaz MR, De S, Freire R, Smits VA, Moldovan GL (2016) HUWE1 interacts with PCNA to alleviate replication stress. EMBO Rep 17(6):874–886. https://doi.org/10.15252/embr.201541685
Welchman RL, Gordon C, Mayer RJ (2005) Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat Rev Mol Cell Biol 6(8):599–609. https://doi.org/10.1038/nrm1700
Vij R, Siegel DS, Jagannath S, Jakubowiak AJ, Stewart AK, McDonagh K, Bahlis N, Belch A, Kunkel LA, Wear S, Wong AF, Wang M (2012) An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. Br J Haematol 158(6):739–748. https://doi.org/10.1111/j.1365-2141.2012.09232.x
Vij R, Wang M, Kaufman JL, Lonial S, Jakubowiak AJ, Stewart AK, Kukreti V, Jagannath S, McDonagh KT, Alsina M, Bahlis NJ, Reu FJ, Gabrail NY, Belch A, Matous JV, Lee P, Rosen P, Sebag M, Vesole DH, Kunkel LA, Wear SM, Wong AF, Orlowski RZ, Siegel DS (2012) An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood 119(24):5661–5670. https://doi.org/10.1182/blood-2012-03-414359
Deshaies RJ, Joazeiro CA (2009) RING domain E3 ubiquitin ligases. Annu Rev Biochem 78:399–434. https://doi.org/10.1146/annurev.biochem.78.101807.093809
Rotin D, Kumar S (2009) Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol 10(6):398–409. https://doi.org/10.1038/nrm2690
Berndsen CE, Wolberger C (2014) New insights into ubiquitin E3 ligase mechanism. Nat Struct Mol Biol 21(4):301–307. https://doi.org/10.1038/nsmb.2780
Kamadurai HB, Qiu Y, Deng A, Harrison JS, Macdonald C, Actis M, Rodrigues P, Miller DJ, Souphron J, Lewis SM, Kurinov I, Fujii N, Hammel M, Piper R, Kuhlman B, Schulman BA (2013) Mechanism of ubiquitin ligation and lysine prioritization by a HECT E3. eLife 2:e00828. https://doi.org/10.7554/eLife.00828
Scheffner M, Nuber U, Huibregtse JM (1995) Protein ubiquitination involving an E1-E2-E3 enzyme ubiquitin thioester cascade. Nature 373(6509):81–83. https://doi.org/10.1038/373081a0
Zhang W, Wu KP, Sartori MA, Kamadurai HB, Ordureau A, Jiang C, Mercredi PY, Murchie R, Hu J, Persaud A, Mukherjee M, Li N, Doye A, Walker JR, Sheng Y, Hao Z, Li Y, Brown KR, Lemichez E, Chen J, Tong Y, Harper JW, Moffat J, Rotin D, Schulman BA, Sidhu SS (2016) System-wide modulation of HECT E3 ligases with selective ubiquitin variant probes. Mol Cell 62(1):121–136. https://doi.org/10.1016/j.molcel.2016.02.005
Lorenz S (2018) Structural mechanisms of HECT-type ubiquitin ligases. Biol Chem 399(2):127–145. https://doi.org/10.1515/hsz-2017-0184
Hodis E, Watson IR, Kryukov GV, Arold ST, Imielinski M, Theurillat JP, Nickerson E, Auclair D, Li L, Place C, Dicara D, Ramos AH, Lawrence MS, Cibulskis K, Sivachenko A, Voet D, Saksena G, Stransky N, Onofrio RC, Winckler W, Ardlie K, Wagle N, Wargo J, Chong K, Morton DL, Stemke-Hale K, Chen G, Noble M, Meyerson M, Ladbury JE, Davies MA, Gershenwald JE, Wagner SN, Hoon DS, Schadendorf D, Lander ES, Gabriel SB, Getz G, Garraway LA, Chin L (2012) A landscape of driver mutations in melanoma. Cell 150(2):251–263. https://doi.org/10.1016/j.cell.2012.06.024
Comprehensive molecular characterization of human colon and rectal cancer (2012). Nature 487 (7407):330–337. https://doi.org/10.1038/nature11252
Li L, Martinez SS, Hu W, Liu Z, Tjian R (2015) A specific E3 ligase/deubiquitinase pair modulates TBP protein levels during muscle differentiation. eLife 4:e08536. doi:https://doi.org/10.7554/eLife.08536
King B, Boccalatte F, Moran-Crusio K, Wolf E, Wang J, Kayembe C, Lazaris C, Yu X, Aranda-Orgilles B, Lasorella A, Aifantis I (2016) The ubiquitin ligase Huwe1 regulates the maintenance and lymphoid commitment of hematopoietic stem cells. Nat Immunol 17(11):1312–1321. https://doi.org/10.1038/ni.3559
Chen LJ, Xu WM, Yang M, Wang K, Chen Y, Huang XJ, Ma QH (2016) HUWE1 plays important role in mouse preimplantation embryo development and the dysregulation is associated with poor embryo development in humans. Sci Rep 6:37928. https://doi.org/10.1038/srep37928
Maghames CM, Lobato-Gil S, Perrin A, Trauchessec H, Rodriguez MS, Urbach S, Marin P, Xirodimas DP (2018) NEDDylation promotes nuclear protein aggregation and protects the ubiquitin proteasome system upon proteotoxic stress. Nat Commun 9(1):4376. https://doi.org/10.1038/s41467-018-06365-0
Bosshard M, Aprigliano R, Gattiker C, Palibrk V, Markkanen E, Backe PH, Pellegrino S, Raymond FL, Froyen G, Altmeyer M, Bjoras M, Dianov GL, van Loon B (2017) Impaired oxidative stress response characterizes HUWE1-promoted X-linked intellectual disability. Sci Rep 7(1):15050. https://doi.org/10.1038/s41598-017-15380-y
The MULE/HUWE1 E3 ubiquitin ligase is a tumor suppressor (2013). Cancer discovery 3 (7):Of32. https://doi.org/10.1158/2159-8290.Cd-rw2013-119
Aqrawi LA, Galtung HK, Guerreiro EM, Ovstebo R, Thiede B, Utheim TP, Chen X, Utheim OA, Palm O, Skarstein K, Jensen JL (2019) Proteomic and histopathological characterisation of sicca subjects and primary Sjogren's syndrome patients reveals promising tear, saliva and extracellular vesicle disease biomarkers. Arthritis Res Ther 21(1):181–114. https://doi.org/10.1186/s13075-019-1961-4
Sander B, Xu W, Eilers M, Popov N, Lorenz S (2017) A conformational switch regulates the ubiquitin ligase HUWE1. eLife 6. https://doi.org/10.7554/eLife.21036
Zhong Q, Gao W, Du F, Wang X (2005) Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell 121(7):1085–1095. https://doi.org/10.1016/j.cell.2005.06.009
Huang L, Kinnucan E, Wang G, Beaudenon S, Howley PM, Huibregtse JM, Pavletich NP (1999) Structure of an E6AP-UbcH7 complex: insights into ubiquitination by the E2-E3 enzyme cascade. Science (New York, NY) 286(5443):1321–1326. https://doi.org/10.1126/science.286.5443.1321
Verdecia MA, Joazeiro CA, Wells NJ, Ferrer JL, Bowman ME, Hunter T, Noel JP (2003) Conformational flexibility underlies ubiquitin ligation mediated by the WWP1 HECT domain E3 ligase. Mol Cell 11(1):249–259
Pandya RK, Partridge JR, Love KR, Schwartz TU, Ploegh HL (2010) A structural element within the HUWE1 HECT domain modulates self-ubiquitination and substrate ubiquitination activities. J Biol Chem 285(8):5664–5673. https://doi.org/10.1074/jbc.M109.051805
Adhikary S, Marinoni F, Hock A, Hulleman E, Popov N, Beier R, Bernard S, Quarto M, Capra M, Goettig S, Kogel U, Scheffner M, Helin K, Eilers M (2005) The ubiquitin ligase HectH9 regulates transcriptional activation by Myc and is essential for tumor cell proliferation. Cell 123(3):409–421. https://doi.org/10.1016/j.cell.2005.08.016
Yanku Y, Bitman-Lotan E, Zohar Y, Kurant E, Zilke N, Eilers M, Orian A (2018) Drosophila HUWE1 ubiquitin ligase regulates Endoreplication and antagonizes JNK signaling during salivary gland development. Cells 7(10). https://doi.org/10.3390/cells7100151
Zhao X, Heng JI, Guardavaccaro D, Jiang R, Pagano M, Guillemot F, Iavarone A, Lasorella A (2008) The HECT-domain ubiquitin ligase Huwe1 controls neural differentiation and proliferation by destabilizing the N-Myc oncoprotein. Nat Cell Biol 10(6):643–653. https://doi.org/10.1038/ncb1727
Zhao Z, Xu D, Wang Z, Wang L, Han R, Wang Z, Liao L, Chen Y (2018) Hepatic PPARalpha function is controlled by polyubiquitination and proteasome-mediated degradation through the coordinated actions of PAQR3 and HUWE1. Hepatology (Baltimore, Md) 68(1):289–303. https://doi.org/10.1002/hep.29786
Herold S, Hock A, Herkert B, Berns K, Mullenders J, Beijersbergen R, Bernards R, Eilers M (2008) Miz1 and HectH9 regulate the stability of the checkpoint protein, TopBP1. EMBO J 27(21):2851–2861. https://doi.org/10.1038/emboj.2008.200
Parsons JL, Tait PS, Finch D, Dianova II, Edelmann MJ, Khoronenkova SV, Kessler BM, Sharma RA, McKenna WG, Dianov GL (2009) Ubiquitin ligase ARF-BP1/Mule modulates base excision repair. EMBO J 28(20):3207–3215. https://doi.org/10.1038/emboj.2009.243
Lee HJ, Li CF, Ruan D, He J, Montal ED, Lorenz S, Girnun GD, Chan CH (2019) Non-proteolytic ubiquitination of hexokinase 2 by HectH9 controls tumor metabolism and cancer stem cell expansion. Nat Commun 10(1):2625. https://doi.org/10.1038/s41467-019-10374-y
Escobar-Henriques M, Joaquim M (2019) Mitofusins: disease gatekeepers and hubs in mitochondrial quality control by E3 ligases. Front Physiol 10:517. https://doi.org/10.3389/fphys.2019.00517
Wu HT, Kuo YC, Hung JJ, Huang CH, Chen WY, Chou TY, Chen Y, Chen YJ, Chen YJ, Cheng WC, Teng SC, Wu KJ (2016) K63-polyubiquitinated HAUSP deubiquitinates HIF-1alpha and dictates H3K56 acetylation promoting hypoxia-induced tumour progression. Nat Commun 7:13644. https://doi.org/10.1038/ncomms13644
Markkanen E, van Loon B, Ferrari E, Parsons JL, Dianov GL, Hubscher U (2012) Regulation of oxidative DNA damage repair by DNA polymerase lambda and MutYH by cross-talk of phosphorylation and ubiquitination. Proc Natl Acad Sci U S A 109(2):437–442. https://doi.org/10.1073/pnas.1110449109
Forget A, Bihannic L, Cigna SM, Lefevre C, Remke M, Barnat M, Dodier S, Shirvani H, Mercier A, Mensah A, Garcia M, Humbert S, Taylor MD, Lasorella A, Ayrault O (2014) Shh signaling protects Atoh1 from degradation mediated by the E3 ubiquitin ligase Huwe1 in neural precursors. Dev Cell 29(6):649–661. https://doi.org/10.1016/j.devcel.2014.05.014
Zhang J, Kan S, Huang B, Hao Z, Mak TW, Zhong Q (2011) Mule determines the apoptotic response to HDAC inhibitors by targeted ubiquitination and destruction of HDAC2. Genes Dev 25(24):2610–2618. https://doi.org/10.1101/gad.170605.111
Yang D, Sun B, Zhang X, Cheng D, Yu X, Yan L, Li L, An S, Jiang H, Lasorella A, Iavarone A, Zhang S, Zou F, Zhao X (2017) Huwe1 sustains Normal ovarian epithelial cell transformation and tumor growth through the histone H1.3-H19 Cascade. Cancer Res 77(18):4773–4784. https://doi.org/10.1158/0008-5472.Can-16-2597
Atsumi Y, Minakawa Y, Ono M, Dobashi S, Shinohe K, Shinohara A, Takeda S, Takagi M, Takamatsu N, Nakagama H, Teraoka H, Yoshioka K (2015) ATM and SIRT6/SNF2H mediate transient H2AX stabilization when DSBs form by blocking HUWE1 to allow efficient gammaH2AX foci formation. Cell Rep 13(12):2728–2740. https://doi.org/10.1016/j.celrep.2015.11.054
Vaughan L, Tan CT, Chapman A, Nonaka D, Mack NA, Smith D, Booton R, Hurlstone AF, Malliri A (2015) HUWE1 ubiquitylates and degrades the RAC activator TIAM1 promoting cell-cell adhesion disassembly, migration, and invasion. Cell Rep 10(1):88–102. https://doi.org/10.1016/j.celrep.2014.12.012
de Groot RE, Ganji RS, Bernatik O, Lloyd-Lewis B, Seipel K, Sedova K, Zdrahal Z, Dhople VM, Dale TC, Korswagen HC, Bryja V (2014) Huwe1-mediated ubiquitylation of dishevelled defines a negative feedback loop in the Wnt signaling pathway. Science signaling 7 (317):ra26. https://doi.org/10.1126/scisignal.2004985
Hall JR, Kow E, Nevis KR, Lu CK, Luce KS, Zhong Q, Cook JG (2007) Cdc6 stability is regulated by the Huwe1 ubiquitin ligase after DNA damage. Mol Biol Cell 18(9):3340–3350. https://doi.org/10.1091/mbc.e07-02-0173
Wang X, Lu G, Li L, Yi J, Yan K, Wang Y, Zhu B, Kuang J, Lin M, Zhang S, Shao G (2014) HUWE1 interacts with BRCA1 and promotes its degradation in the ubiquitin-proteasome pathway. Biochem Biophys Res Commun 444 (4):549–554. doi:https://doi.org/10.1016/j.bbrc.2014.01.075
Noy T, Suad O, Taglicht D, Ciechanover A (2012) HUWE1 ubiquitinates MyoD and targets it for proteasomal degradation. Biochem Biophys Res Commun 418(2):408–413. https://doi.org/10.1016/j.bbrc.2012.01.045
Bernassola F, Karin M, Ciechanover A, Melino G (2008) The HECT family of E3 ubiquitin ligases: multiple players in cancer development. Cancer Cell 14(1):10–21. https://doi.org/10.1016/j.ccr.2008.06.001
Zhang Y, Zhang Y, Xu H (2019) LIMCH1 suppress the growth of lung cancer by interacting with HUWE1 to sustain p53 stability. Gene 712:143963. https://doi.org/10.1016/j.gene.2019.143963
Mandemaker IK, van Cuijk L, Janssens RC, Lans H, Bezstarosti K, Hoeijmakers JH, Demmers JA, Vermeulen W, Marteijn JA (2017) DNA damage-induced histone H1 ubiquitylation is mediated by HUWE1 and stimulates the RNF8-RNF168 pathway. Sci Rep 7(1):15353. https://doi.org/10.1038/s41598-017-15194-y
Di Rita A, Peschiaroli A, D’Acunzo P, Strobbe D, Hu Z, Gruber J, Nygaard M, Lambrughi M, Melino G, Papaleo E, Dengjel J, El Alaoui S, Campanella M, Dotsch V, Rogov VV, Strappazzon F, Cecconi F (2018) HUWE1 E3 ligase promotes PINK1/PARKIN-independent mitophagy by regulating AMBRA1 activation via IKKalpha. Nat Commun 9(1):3755. https://doi.org/10.1038/s41467-018-05722-3
Comprehensive molecular characterization of gastric adenocarcinoma (2014). Nature 513 (7517):202–209. https://doi.org/10.1038/nature13480
Walker BA, Mavrommatis K, Wardell CP, Ashby TC, Bauer M, Davies FE, Rosenthal A, Wang H, Qu P, Hoering A, Samur M, Towfic F, Ortiz M, Flynt E, Yu Z, Yang Z, Rozelle D, Obenauer J, Trotter M, Auclair D, Keats J, Bolli N, Fulciniti M, Szalat R, Moreau P, Durie B, Stewart AK, Goldschmidt H, Raab MS, Einsele H, Sonneveld P, San Miguel J, Lonial S, Jackson GH, Anderson KC, Avet-Loiseau H, Munshi N, Thakurta A, Morgan GJ (2018) Identification of novel mutational drivers reveals oncogene dependencies in multiple myeloma. Blood 132(6):587–597. https://doi.org/10.1182/blood-2018-03-840132
Lin TP, Li J, Li Q, Li X, Liu C, Zeng N, Huang JM, Chu GC, Lin CH, Zhau HE, Chung LWK, Wu BJ, Shih JC (2018) R1 regulates prostate tumor growth and progression by transcriptional suppression of the E3 ligase HUWE1 to stabilize c-Myc. Molec Cancer Res: MCR 16(12):1940–1951. https://doi.org/10.1158/1541-7786.Mcr-16-0346
Yang D, Cheng D, Tu Q, Yang H, Sun B, Yan L, Dai H, Luo J, Mao B, Cao Y, Yu X, Jiang H, Zhao X (2018) HUWE1 controls the development of non-small cell lung cancer through down-regulation of p53. Theranostics 8(13):3517–3529. https://doi.org/10.7150/thno.24401
Mund T, Lewis MJ, Maslen S, Pelham HR (2014) Peptide and small molecule inhibitors of HECT-type ubiquitin ligases. Proc Natl Acad Sci U S A 111(47):16736–16741. https://doi.org/10.1073/pnas.1412152111
Fujita Y, Tinoco R, Li Y, Senft D, Ronai ZA (2019) Ubiquitin ligases in Cancer immunotherapy - balancing antitumor and autoimmunity. Trends Mol Med 25(5):428–443. https://doi.org/10.1016/j.molmed.2019.02.002
Kodama T, Newberg JY, Kodama M, Rangel R, Yoshihara K, Tien JC, Parsons PH, Wu H, Finegold MJ, Copeland NG, Jenkins NA (2016) Transposon mutagenesis identifies genes and cellular processes driving epithelial-mesenchymal transition in hepatocellular carcinoma. Proc Natl Acad Sci U S A 113(24):E3384–E3393. https://doi.org/10.1073/pnas.1606876113
Peter S, Bultinck J, Myant K, Jaenicke LA, Walz S, Muller J, Gmachl M, Treu M, Boehmelt G, Ade CP, Schmitz W, Wiegering A, Otto C, Popov N, Sansom O, Kraut N, Eilers M (2014) Tumor cell-specific inhibition of MYC function using small molecule inhibitors of the HUWE1 ubiquitin ligase. EMBO Mol Med 6(12):1525–1541. https://doi.org/10.15252/emmm.201403927
Myant KB, Cammareri P, Hodder MC, Wills J, Von Kriegsheim A, Gyorffy B, Rashid M, Polo S, Maspero E, Vaughan L, Gurung B, Barry E, Malliri A, Camargo F, Adams DJ, Iavarone A, Lasorella A, Sansom OJ (2017) HUWE1 is a critical colonic tumour suppressor gene that prevents MYC signalling, DNA damage accumulation and tumour initiation. EMBO Molec Medicine 9(2):181–197. https://doi.org/10.15252/emmm.201606684
Funding
This work is funded by the National Natural Science Foundation of China (NO. 81872892 and NO. 81773766), the Natural Science Foundation of Jiangsu Province (NO. BK20181330) and the “Double First-Class” University project (CPU2018GY38).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gong, X., Du, D., Deng, Y. et al. The structure and regulation of the E3 ubiquitin ligase HUWE1 and its biological functions in cancer. Invest New Drugs 38, 515–524 (2020). https://doi.org/10.1007/s10637-020-00894-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-00894-6