Summary
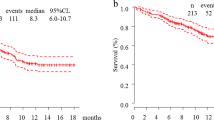
Associations between treatment outcomes of immune checkpoint inhibitors and metastatic sites in advanced non-small cell lung cancer (NSCLC) are not well known. Therefore, this multicenter retrospective study aimed to investigate the predictive factors of metastatic sites after first-line pembrolizumab treatment for advanced NSCLC with a PD-L1 tumor proportion score (TPS) ≥50%. We retrospectively analyzed advanced NSCLC patients with a PD-L1 TPS ≥50% who underwent first-line pembrolizumab therapy at 11 institutions between February 2017 and April 2018. Clinical data collected from medical records included metastatic sites at the time of pembrolizumab treatment. Treatment outcomes of pembrolizumab were assessed according to the Response Evaluation Criteria in Solid Tumors, version 1.1. In total, 213 patients were included in the study. The median age was 71 years (range 39–91 years). Of the 213 patients, 176 (83%) were men and 172 (81%) had an Eastern Cooperative Oncology Group performance status (ECOG-PS) score of 0–1. The most common metastases were thoracic lymph node metastasis (77%), intrapulmonary metastasis (31%), bone metastasis (28%), and malignant pleural effusion (26%). On multivariate analysis, a poor ECOG-PS score (hazard ratio: 1.95, 95.0% confidence interval: 1.25–3.04; P = 0.003) and malignant pleural effusion (hazard ratio: 1.52, 95.0% confidence interval: 1.01–2.29; P = 0.043) were independent predictors of shorter progression-free survival in patients treated with pembrolizumab. For NSCLC patients with malignant pleural effusion, pembrolizumab monotherapy is not a suitable first-line treatment because of its insufficient effectiveness, even though their PD-L1 TPS was high.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30. https://doi.org/10.3322/caac.21387
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA (2008) Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83:584–594. https://doi.org/10.4065/83.5.584
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE et al (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639. https://doi.org/10.1056/NEJMoa1507643
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E et al (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135. https://doi.org/10.1056/NEJMoa1504627
Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY et al (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387:1540–1550. https://doi.org/10.1016/S0140-6736(15)01281-7
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A et al (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833. https://doi.org/10.1056/NEJMoa1606774
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255–265. https://doi.org/10.1016/S0140-6736(16)32517-X
Lopes G, Wu YL, Kudaba I, Kowalski D, Cho BC, Castro G et al (2018) Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 1%: open-label, phase 3 KEYNOTE-042 study. J Clin Oncol 36:LBA4-LBA4. https://doi.org/10.1200/JCO.2018.36.18_suppl.LBA4
Carbognin L, Pilotto S, Milella M, Vaccaro V, Brunelli M, Calio A et al (2015) Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS One 10:e0130142. https://doi.org/10.1371/journal.pone.0130142
Abdel-Rahman O (2016) Correlation between PD-L1 expression and outcome of NSCLC patients treated with anti-PD-1/PD-L1 agents: a meta-analysis. Crit Rev Oncol Hematol 101:75–85. https://doi.org/10.1016/j.critrevonc.2016.03.007
Funazo T, Nomizo T, Kim TH (2017) Liver metastasis is associated with poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. J Thorac Oncol 12:e140–e141. https://doi.org/10.1016/j.jtho.2017.04.027
Kang DH, Chung C, Kim JO, Jung SS, Park HS, Park DI et al (2018) Pleural or pericardial metastasis: a significant factor affecting efficacy and adverse events in lung cancer patients treated with PD-1/PD-L1 inhibitors. Thorac Cancer 9:1500–1508. https://doi.org/10.1111/1759-7714.12877
Schmid S, Diem S, Li Q, Krapf M, Flatz L, Leschka S et al (2018) Organ-specific response to nivolumab in patients with non-small cell lung cancer (NSCLC). Cancer Immunol Immunother 67:1825–1832. https://doi.org/10.1007/s00262-018-2239-4
Shiroyama T, Suzuki H, Tamiya M, Tamiya A, Tanaka A, Okamoto N et al (2018) Clinical characteristics of liver metastasis in nivolumab-treated patients with non-small cell lung cancer. Anticancer Res 38:4723–4729. https://doi.org/10.21873/anticanres.12779
Tamiya M, Tamiya A, Inoue T, Kimura M, Kunimasa K, Nakahama K et al (2018) Metastatic site as a predictor of nivolumab efficacy in patients with advanced non-small cell lung cancer: a retrospective multicenter trial. PLoS One 13:e0192227. https://doi.org/10.1371/journal.pone.0192227
Shibaki S, Murakami S, Shinno Y, Matsumoto Y, Goto Y, Kanda S et al (2019) Malignant pleural effusion as a predictor of the efficacy of anti-PD-1 antibody in patients with non-small cell lung cancer. Thorac Cancer 10:815–822. https://doi.org/10.1111/1759-7714.13004
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Ryu JS, Ryu HJ, Lee SN, Memon A, Lee SK, Nam HS et al (2014) Prognostic impact of minimal pleural effusion in non-small-cell lung cancer. J Clin Oncol 32:960–967. https://doi.org/10.1200/JCO.2013.50.5453
Grove CS, Lee YC (2002) Vascular endothelial growth factor: the key mediator in pleural effusion formation. Curr Opin Pulm Med 8:294–301
Ellis LM, Hicklin DJ (2008) VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 8:579–591. https://doi.org/10.1038/nrc2403
Chouaib S, Messai Y, Couve S, Escudier B, Hasmim M, Noman MZ (2012) Hypoxia promotes tumor growth in linking angiogenesis to immune escape. Front Immunol 3:21. https://doi.org/10.3389/fimmu.2012.00021
Hegde PS, Wallin JJ, Mancao C (2018) Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol 52:117–124. https://doi.org/10.1016/j.semcancer.2017.12.002
Khan KA, Kerbel RS (2018) Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol 15:310–324. https://doi.org/10.1038/nrclinonc.2018.9
Nowell PC (1976) The clonal evolution of tumor cell populations. Science 194:23–28. https://doi.org/10.1126/science.959840
Nahar R, Zhai W, Zhang T, Takano A, Khng AJ, Lee YY et al (2018) Elucidating the genomic architecture of Asian EGFR-mutant lung adenocarcinoma through multi-region exome sequencing. Nat Commun 9:216. https://doi.org/10.1038/s41467-017-02584-z
Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D et al (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366:883–892. https://doi.org/10.1056/NEJMoa1113205
de Bruin EC, McGranahan N, Mitter R, Salm M, Wedge DC, Yates L et al (2014) Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science 346:251–256. https://doi.org/10.1126/science.1253462
Chen J, Jiang CC, Jin L, Zhang XD (2016) Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol 27:409–416. https://doi.org/10.1093/annonc/mdv615
Mansfield AS, Murphy SJ, Peikert T, Yi ES, Vasmatzis G, Wigle DS et al (2016) Heterogeneity of programmed cell death ligand 1 expression in multifocal lung cancer. Clin Cancer Res 22:2177–2182. https://doi.org/10.1158/1078-0432.CCR-15-2246
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078–2092. https://doi.org/10.1056/NEJMoa1801005
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040–2051. https://doi.org/10.1056/NEJMoa1810865
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N et al (2018) Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378:2288–2301. https://doi.org/10.1056/NEJMoa1716948
West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ et al (2019) Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20:924–937. https://doi.org/10.1016/S1470-2045(19)30167-6
Tamiya M, Tamiya A, Yamadori T, Nakao K, Asami K, Yasue T et al (2013) Phase 2 study of bevacizumab with carboplatin-paclitaxel for non-small cell lung cancer with malignant pleural effusion. Med Oncol 30:676. https://doi.org/10.1007/s12032-013-0676-7
Usui K, Sugawara S, Nishitsuji M, Fujita Y, Inoue A, Mouri A et al (2016) A phase II study of bevacizumab with carboplatin-pemetrexed in non-squamous non-small cell lung carcinoma patients with malignant pleural effusions: north East Japan study group trial NEJ013A. Lung Cancer 99:131–136. https://doi.org/10.1016/j.lungcan.2016.07.003
Acknowledgements
We are grateful to all the patients and investigators in this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Drs. Kawachi, Fujimoto, Motohiro Tamiya, Akihiro Tamiya, Hirano, and Kumagai have received lecture fees from Taiho Pharmaceutical Co., Ltd. (Tokyo, Japan) and Merck Sharp & Dohme, Corp. (Tokyo, Japan). Drs. Yokoyama and Hirashima have received lecture fees from Taiho Pharmaceutical Co., Ltd. (Tokyo, Japan). Drs. Ishida and Kanazu have received lecture fees from Merck Sharp & Dohme, Corp. (Tokyo, Japan). All remaining authors have declared no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later aemndments or comparable ethical standards. The study protocol was approved by the review board of each of the 11 institutions and this study was registered with UMIN (University Hospital Medical Information Network in Japan reference number 000032470).
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kawachi, H., Tamiya, M., Tamiya, A. et al. Association between metastatic sites and first-line pembrolizumab treatment outcome for advanced non–small cell lung cancer with high PD-L1 expression: a retrospective multicenter cohort study. Invest New Drugs 38, 211–218 (2020). https://doi.org/10.1007/s10637-019-00882-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00882-5