Summary
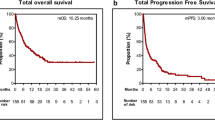
Background It would be useful to have criteria for predicting long-term treatment responses to immune checkpoint inhibitors (ICIs). Maximum depth of response correlates with treatment outcomes among patients receiving programmed death protein 1 axis inhibitors for non-small cell lung cancer (NSCLC). We investigated associations between early depth of response and survival outcomes among patients receiving nivolumab for NSCLC. Methods Using records from prospective observational cohorts, we identified 83 previously treated advanced patients with NSCLC who received nivolumab during 2016–2017. Thirty-one patients who achieved disease control were analyzed. Tumor assessments followed the Response Evaluation Criteria in Solid Tumors (RECIST). Using Kaplan-Meier and receiver operating characteristic (ROC) curve analyses, treatment outcomes were compared with percent tumor reductions from baseline to the first evaluation (8–12 weeks after starting nivolumab). Results Early depth of response was predictive of 6-month progression-free survival (area under the ROC curve, 0.848). Based on ROC results, early tumor shrinkage was defined as a > 10% reduction by the first evaluation. Early tumor shrinkage was associated with significantly longer median progression-free survival (early tumor shrinkage: 16.6 months, 95% confidence interval [CI] 8.5 months–not reached; no early shrinkage: 5.1 months, 95% CI 3.9–6.8 months; P < 0.001) and significantly longer median overall survival (P = 0.046). Conclusions Early depth of tumor shrinkage was associated with outcomes after ICI treatment. Because of its simplicity and predictive ability, early tumor shrinkage may be a promising factor for use in clinical settings. However, confirmation of our results is needed.




Similar content being viewed by others
Abbreviations
- NSCLC:
-
Non-small cell lung cancer
- ICI:
-
Immune checkpoint inhibitor
- PD-1:
-
Programmed death protein 1
- PD-L1:
-
Programmed death ligand 1
- OS:
-
overall survival
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- PFS:
-
Progression-free survival
- EGFR:
-
Epidermal growth factor receptor gene
- TKI:
-
Tyrosine kinase inhibitor
- CT:
-
Computed tomography
- ROC:
-
Receiver operating characteristic
- CI:
-
Confidence interval
- PFS:
-
Progression-free survival
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA (2008) Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83:584–594
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639
Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135
Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387:1540–1550
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823–1833
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, DeMarinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255–265
Ritchie G, Gasper H, Man J, Lord S, Marschner I, Friedlander M, Lee CK (2018) Defining the most appropriate primary end point in phase 2 trials of immune checkpoint inhibitors for advanced solid cancers: a systematic review and meta-analysis. JAMA Oncol 4:522–528
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 11). Eur J Cancer 45:228–247
Shukuya T, Mori K, Amann JM, Bertino EM, Otterson GA, Shields PG, Morita S, Carbone DP (2016) Relationship between overall survival and response or progression-free survival in advanced non-small cell lung cancer patients treated with anti-PD-1/PD-L1 antibodies. J Thorac Oncol 11:1927–1939
Nishino M, Dahlberg SE, Adeni AE, Lydon CA, Hatabu H, Janne PA, Hodi FS, Awad MM (2017) Tumor response dynamics of advanced non-small cell lung cancer patients treated with pd-1 inhibitors: imaging markers for treatment outcome. Clin Cancer Res 23:5737–5744
McCoach CE, Blumenthal GM, Zhang L, Myers A, Tang S, Sridhara R, Keegan P, Pazdur R, Doebele RC, Kazandjian D (2017) Exploratory analysis of the association of depth of response and survival in patients with metastatic non-small-cell lung cancer treated with a targeted therapy or immunotherapy. Ann Oncol 28:2707–2714
Takeda M, Okamoto I, Nakagawa K (2014) Survival outcome assessed according to tumor response and shrinkage pattern in patients with EGFR mutation-positive non-small-cell lung cancer treated with gefitinib or erlotinib. J Thorac Oncol 9:200–204
Lee CK, Lord S, Marschner I, Wu YL, Sequist L, Rosell R, Fukuoka M, Mitsudomi T, Asher R, Davies L, Gebski V, Gralla R, Mok T, Chih-Hsin Yang J (2018) The value of early depth of response in predicting long-term outcome in EGFR-mutant lung cancer. J Thorac Oncol 13:792–800
Fujimoto D, Yoshioka H, Kataoka Y, Morimoto T, Kim YH, Tomii K, Ishida T, Hirabayashi M, Hara S, Ishitoko M, Fukuda Y, Hwang MH, Sakai N, Fukui M, Nakaji H, Morita M, Mio T, Yasuda T, Sugita T, Hirai T (2018) Efficacy and safety of nivolumab in previously treated patients with non-small cell lung cancer: a multicenter retrospective cohort study. Lung Cancer 119:14–20
Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, Poddubskaya E, Borghaei H, Felip E, Paz-Ares L, Pluzanski A, Reckamp KL, Burgio MA, Kohlhaeufl M, Waterhouse D, Barlesi F, Antonia S, Arrieta O, Fayette J, Crino L, Rizvi N, Reck M, Hellmann MD, Geese WJ, Li A, Blackwood-Chirchir A, Healey D, Brahmer J, WEberhardt EE (2017) Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase iii trials (CheckMate 017 and CheckMate 057). J Thorac Oncol 35:3924–3933
Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, Patnaik A, Ribas A, Robert C, Gangadhar TC, Joshua AM, Hersey P, Dronca R, Joseph R, Hille D, Xue D, Li XN, Kang SP, Ebbinghaus S, Perrone A, Wolchok JD (2016) Evaluation of immune-related response criteria and RECIST v11 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 34:1510–1517
Nishino M, Giobbie-Hurder A, Manos MP, Bailey N, Buchbinder EI, Ott PA, Ramaiya NH, Hodi FS (2017) Immune-related tumor response dynamics in melanoma patients treated with pembrolizumab: identifying markers for clinical outcome and treatment decisions. Clin Cancer Res 23:4671–4679
Nishino M, Tirumani SH, Ramaiya NH, Hodi FS (2015) Cancer immunotherapy and immune-related response assessment: the role of radiologists in the new arena of cancer treatment. Eur J Radiol 84:1259–1268
Chiou VL, Burotto M (2015) Pseudoprogression and immune-related response in solid tumors. J Clin Oncol 33:3541–3543
Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15:7412–7420
Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, Humphrey R, Blumenstein B, Old L, Wolchok J (2010) Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 102:1388–1397
Katz SI, Hammer M, Bagley SJ, Aggarwal C, Bauml JM, Thompson JC, Nachiappan AC, Simone CB, Langer CJ (2018) Radiologic pseudoprogression during anti-PD-1 therapy for advanced non-small cell lung cancer. J Thorac Oncol 13:978–986
Acknowledgements
The authors thank Keiko Sakuragawa for her administrative assistance.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of potential conflicts of interest
Dr. Kawachi has received lecture fees from Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical, Merck Sharp and Dohme, and Taiho Pharmaceutical. Dr. Fujimoto has received lecture fees from Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb K.K., Chugai Pharmaceutical, AstraZeneca, Merck Sharp and Dohme, and Taiho Pharmaceutical. Dr. Hosoya has received lecture fees from Chugai Pharmaceutical, Merck Sharp and Dohme, and Taiho Pharmaceutical. Dr. Sato has received lecture fees from Ono Pharmaceutical Co., Ltd. Dr. Tomii has received lecture fees from Chugai Pharmaceutical, AstraZeneca, and Taiho Pharmaceutical. The remaining authors have declared that they have no conflicts of interest.
Ethical approval
All study procedures complied with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Additional information
Summary headings
• RECIST-based tumor shrinkage may not predict outcomes from ICI treatment.
• A > 10% decrease in tumor size predicted good outcomes from nivolumab.
• Early tumor shrinkage may be a simple and useful marker in the clinical setting.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Online Resource 1
Supplementary Figure Kaplan-Meier curves for progression-free survival among patients with stable disease at the first evaluation (N = 19). The survival analysis is stratified by early tumor shrinkage (present vs. absent). Each curve is labelled with the median survival time, the total number events, and the total number of patients (PDF 109 kb)
Rights and permissions
About this article
Cite this article
Kawachi, H., Fujimoto, D., Morimoto, T. et al. Early depth of tumor shrinkage and treatment outcomes in non-small cell lung cancer treated using Nivolumab. Invest New Drugs 37, 1257–1265 (2019). https://doi.org/10.1007/s10637-019-00770-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00770-y