Summary
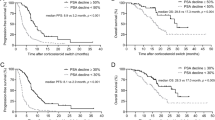
The aim of this retrospective study is to evaluate the activity and safety of a steroidal switch from prednisone to dexamethasone in patients with advanced, heavily pre-treated, castration-resistant prostate cancer (CRPC) who progressed on abiraterone acetate. Treatment consisted of oral daily abiraterone plus dexamethasone (0.5 mg once daily) administered until disease progression or unacceptable toxicity. Thirty-six patients were evaluated: all men underwent a prior treatment with enzalutamide. A PSA decrease ≥50% was observed in 11% of patients; median progression-free survival was 10.8 weeks (95% CI: 9.2–16), and median survival was 17.6 weeks (95% CI: 15.8–28.8). Better efficacy and survival were observed in the subgroup of patients treated with abiraterone acetate prior for a period >3 months; treatment was well tolerated, and no grade 3–4 toxicities were observed. Our findings did not suggest the use of steroid switch in all CRPC who were heavily pre-treated. However, the switch could be an option for patients who responded well to prior abiraterone acetate treatment.


Similar content being viewed by others
References
Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, Gavin A, Visser O, Bray F (2018) Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer
Komura K, Sweeney CJ, Inamoto T, Ibuki N, Azuma H, Kantoff PW (2017) Current treatment strategies for advanced prostate cancer. Int J Urol.
Xu L, Pachynski RK (2018) Contemporary Management of the Newly Diagnosed Prostate Cancer Patient with Metastatic Disease at Presentation. Curr Urol Rep. 19(10):79
Starosta SB, Savage SJ (2018) Castration-Resistant Prostate Cancer: Sequencing Oral and Infusion Agents. Curr Urol Rep. 19(9):73
Efstathiou E, Titus M, Tsavachidou D, Tzelepi V, Wen S, Hoang A et al (2012) Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol. 30:637–643
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L et al (2011) Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364:1995–2005
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P et al (2013) Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 368:138–148
Caffo O, De Giorgi U, Fratino L, Alesini D, Zagonel V, Facchini G et al (2015) Clinical Outcomes of Castration-resistant Prostate Cancer Treatments Administered as Third or Fourth Line Following Failure of Docetaxel and Other Second-line Treatment: Results of an Italian Multicentre Study. Eur Urol. 68(1):147–153
Buonerba C, Federico P, Bosso D, Puglia L, Policastro T, Izzo M et al (2014) Carboplatin plus etoposide in heavily pretreated castration-resistant prostate cancer patients. Future Oncol. 10(8):1353–1360
Petrioli R, Roviello G, Fiaschi AI, Laera L, Bianco V, Ponchietti R et al (2015) Low-Dose Estramustine Phosphate and Concomitant Low-Dose Acetylsalicylic Acid in Heavily Pretreated Patients With Advanced Castration-Resistant Prostate Cancer. Clin Genitourin Cancer. 13(5):441–446
Petrioli R, Roviello G, Fiaschi AI, Laera L, Miano ST, De Rubertis G et al (2015) Rechallenge of docetaxel combined with epirubicin given on a weekly schedule in advanced castration-resistant prostate cancer patients previously exposed to docetaxel and abiraterone acetate: a single-institution experience. Med Oncol. 32(3):52
Venkitaraman R (2015) Lorente D,Murthy V et al. A randomised phase 2 trial of dexamethasone versus prednisolone in castration-resistant prostate cancer. Eur Urol 67:673–679
Lorente D, Omlin A, Ferraldeschi R et al (2014) Tumour responses following a steroid switch from prednisone to dexamethasone in castration-resistant prostate cancer patients progressing on abiraterone. Br J Cancer 111:2248–2253
Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M et al (1999) Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 17:3461–3467
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA et al (2008) Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 26:1148–1159
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. J45(2):228–247
Melzack R (1975) The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1:277–299
Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0. Published August 9, 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_30 (accessed Sept 14, 2010).
Francini E, Petrioli R, Roviello G (2014) No clear evidence of a clinical benefit of a sequential therapy regimen with abiraterone acetate and enzalutamide. Expert Rev Anticancer Ther. 19:1–6
Mostaghel EA (2014) Abiraterone in the treatment of metastatic castration-resistant prostate cancer. Cancer Manag Res. 6:39–51
Roviello G, Sigala S, Danesi R, Re MD, Bonetta A, Cappelletti MR, Zanotti L, Bottini A, Generali D (2016) Incidence and relative risk of adverse events of special interest in patients with castration resistant prostate cancer treated with CYP-17 inhibitors: A meta-analysis of published trials. Crit Rev Oncol Hematol. 101:12–20. https://doi.org/10.1016/j.critrevonc.2016.02.013. Epub 2016 Feb 27 Review
Arora V, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, Shah N, Cai L, Efstathiou E, Logothetis C, Zhend D, Sawyers CL ((2013)) Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 155(6):1309–1322
Dizdar O (2015) Is dexamethasone a better partner for abiraterone than prednisolone? Oncologist. 20(5):e13
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guideline). Prostate Cancer. Version 2.2014. Available at: http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed July 13, 2013.
Horwich A, Hugosson J, de Reijke T, Wiegel T, Fizazi K, Kataja V (2013) Panel Members; European Society for Medical Oncology. Prostate cancer: ESMO Consensus Conference Guidelines 2012. Ann Oncol. 24:1141–1162
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Roviello G declares that he has no conflicts of interest. Petrioli R declares that he has no conflicts of interest. Bonetta A declares that he has no conflicts of interest. Conca R declares that he has no conflicts of interest. Rosellini P declares that she has no conflicts of interest. Aieta M declares that he has no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
When possible, informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Roviello, G., Petrioli, R., Bonetta, A. et al. Corticosteroid switch in heavily pre-treated castration-resistant prostate cancer patients progressed on abiraterone acetate plus prednisone. Invest New Drugs 36, 1110–1115 (2018). https://doi.org/10.1007/s10637-018-0685-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0685-7