Summary
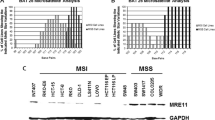
Intended to explore synthetic lethality and develop better combinatorial regimens, we screened colorectal cancer (CRC) cells using poly ADP-ribose (PAR) polymerase (PARP) inhibitors and cytotoxic agents. We studied four PARP inhibitors and three DNA-damaging agents, and their combinations using sulforhodamine B assay. Rucaparib demonstrated the greatest synergy with irinotecan, followed by olaparib and PJ34. Rucaparib and irinotecan was further subjected to detailed examination to determine combination index (CI) and underlying mechanism of action. Effectiveness and sequence dependence of this combination were assessed in microsatellite stable (MSS) and unstable (MSI) CRC and HCT116 isogenic cell lines. The degree of cell cycle arrest and apoptosis was determined by FACS. In vivo studies were performed to confirm efficacy of this combination. PAR levels in MSI and PARP expression in MSI and MSS cell lines were diminished upon combinatorial treatment. HCT116 isogenic cells revealed the importance of p21, p53 and PTEN in exerting synergy. In MSI cells, administration of rucaparib prior to irinotecan enhanced cytotoxicity compared to other strategies explored. FACS revealed S-phase arrest and increased late-stage apoptosis in MSS, and G2-M arrest and total and early-stage apoptosis in MSI cells. In in vivo murine xenograft models, a significant reduction in tumor volume and expression of Ki67, pancytokeratin and RPS6KB1, and increase in expression of caspase 3 were observed with the combination. In conclusion, among the various combinations studied, rucaparib plus irinotecan was the most synergistic one. Alterations in cell cycle arrest and apoptosis were dependent on MSI status in CRC cells.






Similar content being viewed by others
References
Lin YL, Liau JY, Yu SC, Ou DL, Lin LI, Tseng LH, Chang YL, Yeh KH, Cheng AL (2012) KRAS mutation is a predictor of oxaliplatin sensitivity in colon cancer cells. PLoS One 7(11):e50701. https://doi.org/10.1371/journal.pone.0050701
Peters GJ (2015) Therapeutic potential of TAS-102 in the treatment of gastrointestinal malignancies. Ther Adv Med Oncol 7(6):340–356. https://doi.org/10.1177/1758834015603313
Platell C, Ng S, O'Bichere A, Tebbutt N (2011) Changing management and survival in patients with stage IV colorectal cancer. Dis Colon Rectum 54(2):214–219. https://doi.org/10.1007/DCR.0b013e3182023bb0
Rabik CA, Dolan ME (2007) Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev 33(1):9–23. https://doi.org/10.1016/j.ctrv.2006.09.006
Osoegawa A, Gills JJ, Kawabata S, Dennis PA (2017) Rapamycin sensitizes cancer cells to growth inhibition by the PARP inhibitor olaparib. Oncotarget 8(50):87044–87053. https://doi.org/10.18632/oncotarget.19667
Wei H, Yu X (2016) Functions of PARylation in DNA damage repair pathways. Genomics Proteomics Bioinformatics 14(3):131–139. https://doi.org/10.1016/j.gpb.2016.05.001
Dizdar O, Arslan C, Altundag K (2015) Advances in PARP inhibitors for the treatment of breast cancer. Expert Opin Pharmacother 16(18):2751–2758. https://doi.org/10.1517/14656566.2015.1100168
Munoz-Gamez JA, Martin-Oliva D, Aguilar-Quesada R, Canuelo A, Nunez MI, Valenzuela MT, Ruiz de Almodovar JM, De Murcia G, Oliver FJ (2005) PARP inhibition sensitizes p53-deficient breast cancer cells to doxorubicin-induced apoptosis. Biochem J 386(Pt 1):119–125. https://doi.org/10.1042/BJ20040776
Walsh C (2018) Targeted therapy for ovarian cancer: the rapidly evolving landscape of PARP inhibitor use. Minerva Ginecol 70(2):150–170. https://doi.org/10.23736/S0026-4784.17.04152-1
Foucquier J, Guedj M (2015) Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect 3(3):e00149. https://doi.org/10.1002/prp2.149
Murai J, Zhang Y, Morris J, Ji J, Takeda S, Doroshow JH, Pommier Y (2014) Rationale for poly(ADP-ribose) polymerase (PARP) inhibitors in combination therapy with camptothecins or temozolomide based on PARP trapping versus catalytic inhibition. J Pharmacol Exp Ther 349(3):408–416. https://doi.org/10.1124/jpet.113.210146
Augustine TA, Baig M, Sood A, Budagov T, Atzmon G, Mariadason JM, Aparo S, Maitra R, Goel S (2015) Telomere length is a novel predictive biomarker of sensitivity to anti-EGFR therapy in metastatic colorectal cancer. Br J Cancer 112(2):313–318. https://doi.org/10.1038/bjc.2014.561
Gandhi JS, Goswami M, Sharma A, Tanwar P, Gupta G, Gupta N, Pasricha S, Mehta A, Singh S, Agarwal M, Gupta N (2017) Clinical impact of mismatch repair protein testing on outcome of early staged colorectal carcinomas. J Gastrointest Cancer 49:406–414. https://doi.org/10.1007/s12029-017-9954-5
Shirasawa S, Furuse M, Yokoyama N, Sasazuki T (1993) Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science 260(5104):85–88
Samuels Y, Diaz LA Jr, Schmidt-Kittler O, Cummins JM, Delong L, Cheong I, Rago C, Huso DL, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE (2005) Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell 7(6):561–573. https://doi.org/10.1016/j.ccr.2005.05.014
Jhawer M, Goel S, Wilson AJ, Montagna C, Ling YH, Byun DS, Nasser S, Arango D, Shin J, Klampfer L, Augenlicht LH, Perez-Soler R, Mariadason JM (2008) PIK3CA mutation/PTEN expression status predicts response of colon cancer cells to the epidermal growth factor receptor inhibitor cetuximab. Cancer Res 68(6):1953–1961. https://doi.org/10.1158/0008-5472.CAN-07-5659
Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, Jeffrey SS, Van de Rijn M, Waltham M, Pergamenschikov A, Lee JC, Lashkari D, Shalon D, Myers TG, Weinstein JN, Botstein D, Brown PO (2000) Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet 24(3):227–235. https://doi.org/10.1038/73432
Samaraweera L, Adomako A, Rodriguez-Gabin A, McDaid HM (2017) A novel indication for panobinostat as a senolytic drug in NSCLC and HNSCC. Sci Rep 7(1):1900. https://doi.org/10.1038/s41598-017-01964-1
Chou TC (2010) Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70(2):440–446. https://doi.org/10.1158/0008-5472.CAN-09-1947
Qin Y, Qi N, Tang Y, He J, Li X, Gu F, Zou S (2015) Isolation and identification of a high molecular weight protein in sow milk. Animal 9(5):847–854. https://doi.org/10.1017/S1751731114003280
Palma JP, Rodriguez LE, Bontcheva-Diaz VD, Bouska JJ, Bukofzer G, Colon-Lopez M, Guan R, Jarvis K, Johnson EF, Klinghofer V, Liu X, Olson A, Saltarelli MJ, Shi Y, Stavropoulos JA, Zhu GD, Penning TD, Luo Y, Giranda VL, Rosenberg SH, Frost DJ, Donawho CK (2008) The PARP inhibitor, ABT-888 potentiates temozolomide: correlation with drug levels and reduction in PARP activity in vivo. Anticancer Res 28(5A):2625–2635
Brookes S, Gagrica S, Sanij E, Rowe J, Gregory FJ, Hara E, Peters G (2015) Evidence for a CDK4-dependent checkpoint in a conditional model of cellular senescence. Cell Cycle 14(8):1164–1173. https://doi.org/10.1080/15384101.2015.1010866
Vermes I, Haanen C, Reutelingsperger C (2000) Flow cytometry of apoptotic cell death. J Immunol Methods 243(1–2):167–190
He BC, Gao JL, Luo X, Luo J, Shen J, Wang L, Zhou Q, Wang YT, Luu HH, Haydon RC, Wang CZ, Du W, Yuan CS, He TC, Zhang BQ (2011) Ginsenoside Rg3 inhibits colorectal tumor growth through the down-regulation of Wnt/ss-catenin signaling. Int J Oncol 38(2):437–445. https://doi.org/10.3892/ijo.2010.858
Xiao K, Luo J, Fowler WL, Li Y, Lee JS, Xing L, Cheng RH, Wang L, Lam KS (2009) A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials 30(30):6006–6016. https://doi.org/10.1016/j.biomaterials.2009.07.015
Zhao E, Ilyas G, Cingolani F, Choi JH, Ravenelle F, Tanaka KE, Czaja MJ (2017) Pentamidine blocks hepatotoxic injury in mice. Hepatology 66(3):922–935. https://doi.org/10.1002/hep.29244
Hammond WA, Swaika A, Mody K (2016) Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol 8(1):57–84. https://doi.org/10.1177/1758834015614530
Bradshaw-Pierce EL, Pitts TM, Kulikowski G, Selby H, Merz AL, Gustafson DL, Serkova NJ, Eckhardt SG, Weekes CD (2013) Utilization of quantitative in vivo pharmacology approaches to assess combination effects of everolimus and irinotecan in mouse xenograft models of colorectal cancer. PLoS One 8(3):e58089. https://doi.org/10.1371/journal.pone.0058089
Tsukihara H, Nakagawa F, Sakamoto K, Ishida K, Tanaka N, Okabe H, Uchida J, Matsuo K, Takechi T (2015) Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102, together with bevacizumab, cetuximab, or panitumumab on human colorectal cancer xenografts. Oncol Rep 33(5):2135–2142. https://doi.org/10.3892/or.2015.3876
Vormoor B, Curtin NJ (2014) Poly(ADP-ribose) polymerase inhibitors in Ewing sarcoma. Curr Opin Oncol 26(4):428–433. https://doi.org/10.1097/CCO.0000000000000091
Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, Ji J, Takeda S, Pommier Y (2012) Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res 72(21):5588–5599. https://doi.org/10.1158/0008-5472.CAN-12-2753
Noll DM, Mason TM, Miller PS (2006) Formation and repair of interstrand cross-links in DNA. Chem Rev 106(2):277–301. https://doi.org/10.1021/cr040478b
Nicolay NH, Ruhle A, Perez RL, Trinh T, Sisombath S, Weber KJ, Schmezer P, Ho AD, Debus J, Saffrich R, Huber PE (2016) Mesenchymal stem cells exhibit resistance to topoisomerase inhibition. Cancer Lett 374(1):75–84. https://doi.org/10.1016/j.canlet.2016.02.007
Abdou I, Poirier GG, Hendzel MJ, Weinfeld M (2015) DNA ligase III acts as a DNA strand break sensor in the cellular orchestration of DNA strand break repair. Nucleic Acids Res 43(2):875–892. https://doi.org/10.1093/nar/gku1307
Stewart E, Goshorn R, Bradley C, Griffiths LM, Benavente C, Twarog NR, Miller GM, Caufield W, Freeman BB 3rd, Bahrami A, Pappo A, Wu J, Loh A, Karlstrom A, Calabrese C, Gordon B, Tsurkan L, Hatfield MJ, Potter PM, Snyder SE, Thiagarajan S, Shirinifard A, Sablauer A, Shelat AA, Dyer MA (2014) Targeting the DNA repair pathway in Ewing sarcoma. Cell Rep 9(3):829–841. https://doi.org/10.1016/j.celrep.2014.09.028
Cao TP, Kim JS, Woo MH, Choi JM, Jun Y, Lee KH, Lee SH (2016) Structural insight for substrate tolerance to 2-deoxyribose-5-phosphate aldolase from the pathogen Streptococcus suis. J Microbiol 54(4):311–321. https://doi.org/10.1007/s12275-016-6029-4
Strumberg D, Pilon AA, Smith M, Hickey R, Malkas L, Pommier Y (2000) Conversion of topoisomerase I cleavage complexes on the leading strand of ribosomal DNA into 5′-phosphorylated DNA double-strand breaks by replication runoff. Mol Cell Biol 20(11):3977–3987
Malanga M, Althaus FR (2004) Poly(ADP-ribose) reactivates stalled DNA topoisomerase I and induces DNA strand break resealing. J Biol Chem 279(7):5244–5248. https://doi.org/10.1074/jbc.C300437200
Sugimura K, Takebayashi S, Taguchi H, Takeda S, Okumura K (2008) PARP-1 ensures regulation of replication fork progression by homologous recombination on damaged DNA. J Cell Biol 183(7):1203–1212. https://doi.org/10.1083/jcb.200806068
Marechal A, Zou L (2013) DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol 5(9). https://doi.org/10.1101/cshperspect.a012716
Kaku Y, Tsuchiya A, Kanno T, Nishizaki T (2015) Irinotecan induces cell cycle arrest, but not apoptosis or necrosis, in Caco-2 and CW2 colorectal cancer cell lines. Pharmacology 95(3–4):154–159. https://doi.org/10.1159/000381029
Williams AB, Schumacher B (2016) p53 in the DNA-damage-repair process. Cold Spring Harb Perspect Med 6(5). https://doi.org/10.1101/cshperspect.a026070
Origanti S, Cai SR, Munir AZ, White LS, Piwnica-Worms H (2013) Synthetic lethality of Chk1 inhibition combined with p53 and/or p21 loss during a DNA damage response in normal and tumor cells. Oncogene 32(5):577–588. https://doi.org/10.1038/onc.2012.84
Abd Elmageed ZY, Naura AS, Errami Y, Zerfaoui M (2012) The poly(ADP-ribose) polymerases (PARPs): new roles in intracellular transport. Cell Signal 24(1):1–8. https://doi.org/10.1016/j.cellsig.2011.07.019
Davidson D, Wang Y, Aloyz R, Panasci L (2013) The PARP inhibitor ABT-888 synergizes irinotecan treatment of colon cancer cell lines. Investig New Drugs 31(2):461–468. https://doi.org/10.1007/s10637-012-9886-7
Lieberman HB, Panigrahi SK, Hopkins KM, Wang L, Broustas CG (2017) p53 and RAD9, the DNA damage response, and regulation of transcription networks. Radiat Res 187(4):424–432. https://doi.org/10.1667/RR003CC.1
Rosado MM, Bennici E, Novelli F, Pioli C (2013) Beyond DNA repair, the immunological role of PARP-1 and its siblings. Immunology 139(4):428–437. https://doi.org/10.1111/imm.12099
Lee YC, Lee CH, Tsai HP, An HW, Lee CM, Wu JC, Chen CS, Huang SH, Hwang J, Cheng KT, Leiw PL, Chen CL, Lin CM (2015) Targeting of topoisomerase I for prognoses and therapeutics of camptothecin-resistant ovarian cancer. PLoS One 10(7):e0132579. https://doi.org/10.1371/journal.pone.0132579
Huang K, Zhang J, O'Neill KL, Gurumurthy CB, Quadros RM, Tu Y, Luo X (2016) Cleavage by caspase 8 and mitochondrial membrane association activate the BH3-only protein bid during TRAIL-induced apoptosis. J Biol Chem 291(22):11843–11851. https://doi.org/10.1074/jbc.M115.711051
McIlwain DR, Berger T, Mak TW (2015) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 7(4). https://doi.org/10.1101/cshperspect.a026716
Mao D, Qiao L, Lu H, Feng Y (2016) B-cell translocation gene 3 overexpression inhibits proliferation and invasion of colorectal cancer SW480 cells via Wnt/beta-catenin signaling pathway. Neoplasma 63(5):705–716. https://doi.org/10.4149/neo_2016_507
Melling N, Kowitz CM, Simon R, Bokemeyer C, Terracciano L, Sauter G, Izbicki JR, Marx AH (2016) High Ki67 expression is an independent good prognostic marker in colorectal cancer. J Clin Pathol 69(3):209–214. https://doi.org/10.1136/jclinpath-2015-202985
Al-Ali H, Ding Y, Slepak T, Wu W, Sun Y, Martinez Y, Xu XM, Lemmon VP, Bixby JL (2017) The mTOR substrate S6 kinase 1 (S6K1) is a negative regulator of axon regeneration and a potential drug target for central nervous system injury. J Neurosci 37(30):7079–7095. https://doi.org/10.1523/JNEUROSCI.0931-17.2017
Therkildsen C, Bergmann TK, Henrichsen-Schnack T, Ladelund S, Nilbert M (2014) The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: a systematic review and meta-analysis. Acta Oncol 53(7):852–864. https://doi.org/10.3109/0284186X.2014.895036
Satelli A, Mitra A, Brownlee Z, Xia X, Bellister S, Overman MJ, Kopetz S, Ellis LM, Meng QH, Li S (2015) Epithelial-mesenchymal transitioned circulating tumor cells capture for detecting tumor progression. Clin Cancer Res 21(4):899–906. https://doi.org/10.1158/1078-0432.CCR-14-0894
Acknowledgements
S. Goel is supported by a K-12 award from the National Cancer Institute of the National Institutes of Health 1K12CA132783-01A1, and an Advanced Clinical Research Award (ACRA) in colon cancer, by the ASCO (now Conquer) Cancer Foundation. The authors would like to thank Dr. Tanya Dragic from Department of Microbiology & Immunology, Dr. Balazs Halmos from Department of Medicine and Dr. Thomas J. Ow from Department of Pathology, Albert Einstein College of Medicine/Montefiore Medical Center for their helpful advice on various technical issues examined in this paper, and Dr. Dhanonjoy C. Saha from Office of Grant Support, Albert Einstein College of Medicine, for his advice and comments. The authors also greatly appreciate the expert advice/assistance of Hillary Guzik, Analytical Imaging Facility, Albert Einstein College of Medicine in microscopy/IHC studies. The imaging was conducted in the Analytical Imaging Facility, which is funded by the NCI Cancer Grant P30CA013330.
Funding
The work was supported by the K-12 award from the National Cancer Institute of the National Institutes of Health 1K12CA132783-01A1, and an Advanced Clinical Research Award (ACRA) in colon cancer, by the ASCO (now Conquer) Cancer Foundation to Dr. Sanjay Goel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Titto Augustine declares that he has no conflict of interest. Radhashree Maitra declares that she has no conflict of interest. Jinghang Zhang declares that she has no conflict of interest. Jay Nayak declares that he has no conflict of interest. Sanjay Goel declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants. All applicable institutional guidelines (by Institutional Animal Care and Use Committee) for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Augustine, T., Maitra, R., Zhang, J. et al. Sensitization of colorectal cancer to irinotecan therapy by PARP inhibitor rucaparib. Invest New Drugs 37, 948–960 (2019). https://doi.org/10.1007/s10637-018-00717-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-00717-9