Summary
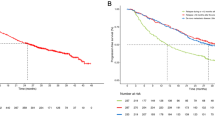
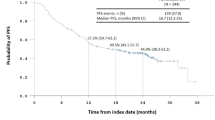
Background Cisplatin and pemetrexed are very effective against advanced non-squamous non-small cell lung cancer (NSCLC) without EGFR mutations. Erlotinib plus bevacizumab are highly effective against advanced NSCLCs with activating EGFR mutations. We performed this phase I ‘Quartet Trial’ to determine the safety and efficacy of all 4 agents as a first-line treatment for non-squamous NSCLC patients harboring activating EGFR mutations. Patients and Methods Patients received escalating quartet-agent doses every 3 weeks for 4 cycles. We examined the dose-limiting toxicity (DLT) to determine the maximum tolerated dose (MTD) and recommended dose (RD). Results Ten patients (3 men and 7 women) with a median age of 69 years were enrolled. Four and 6 patients had exon 19 and 21 mutations, respectively; 8 received maintenance therapy without unexpected or cumulative toxicities. One of 6 patients experienced grade 3 vagal reflex at 60 mg/m2 cisplatin plus 500 mg/m2 pemetrexed with 150 mg erlotinib and 15 mg/kg bevacizumab, which was designated the RD. Four patients experienced no DLT with 75 mg/m2 cisplatin plus 500 mg/m2 pemetrexed with 150 mg erlotinib and 15 mg/kg bevacizumab (designated the MTD); however, 3 underwent dose reduction due to severe toxicities (grade 3 gastrointestinal hemorrhage, skin rash, nausea, and febrile neutropenia) during induction chemotherapy. The most frequent DLT-phase adverse events were nausea, anorexia, and fatigue. The overall response rate was 100%. Furthermore, the progression-free and overall survival rates were 17.9 and 32.0 months, respectively. Conclusions This quartet chemotherapy regimen was tolerable and effective in our patient population (UMIN000012536).

Similar content being viewed by others
References
Hoffman PC, Mauer AM, Vokes EE (2000) Lung cancer. Lancet 355:479–485
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH, Eastern Cooperative Oncology Group (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92–98
Breathnach OS, Freidlin B, Conley B, Green MR, Johnson DH, Gandara DR, O'Connell M, Shepherd FA, Johnson BE (2001) Twenty-two years of phase III trials for patients with advanced non-small-cell lung cancer: sobering results. J Clin Oncol 19:1734–1742
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350:2129–2139
Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H (2007) Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448:561–566
Sandler AB, Johnson DH, Herbst RS (2004) Anti-vascular endothelial growth factor monoclonals in non-small cell lung cancer. Clin Cancer Res 10:4258s–4262s
Midha A, Dearden S, McCormack R (2015) EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res 5:2892–2911
Tamiya A, Tamiya M, Shiroyama T, Saijo N, Nakatani T, Minomo S, Tsuji T, Takeuchi N, Omachi N, Kurata K, Suzuki H, Okamoto N, Okishio K, Hirashima T, Atagi S (2015) Phase II trial of carboplatin, S-1, and gefitinib as first-line triplet chemotherapy for advanced non-small cell lung cancer patients with activating epidermal growth factor receptor mutations. Med Oncol 32:40
Sugawara S, Oizumi S, Minato K, Harada T, Inoue A, Fujita Y, Maemondo M, Yoshizawa H, Ito K, Gemma A, Nishitsuji M, Harada M, Isobe H, Kinoshita I, Morita S, Kobayashi K, Hagiwara K, Kurihara M, Nukiwa T, North East Japan Study Group and Tokyo Cooperative Oncology Group (2015) Randomized phase II study of concurrent versus sequential alternating gefitinib and chemotherapy in previously untreated non-small cell lung cancer with sensitive EGFR mutations: NEJ005/TCOG0902. Ann Oncol 26:888–894
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowalti A, Lilenbaum R, Johnson DH (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C (2009) Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 27:1227–1234
Barlesi F, Scherpereel A, Gorbunova V, Gervais R, Vikström A, Chouaid C, Chella A, Kim JH, Ahn MJ, Reck M, Pazzola A, Kim HT, Aerts JG, Morando C, Loundou A, Groen HJ, Rittmeyer A (2014) Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann Oncol 25:1044–1052
Herbst RS, Ansari R, Bustin F, Flynn P, Hart L, Otterson GA, Vlahovic G, Soh CH, O'Connor P, Hainsworth J (2011) Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet 377:1846–1854
Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, Yamamoto N, Hida T, Maemondo M, Nakagawa K, Nagase S, Okamoto I, Yamanaka T, Tajima K, Harada R, Fukuoka M, Yamamoto N (2014) Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 15:1236–1244
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T, North-East Japan Study Group (2010) Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 362:2380–2388
Rosell R, Carcereny E, Gervais R et al (2012) Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 13:239–246
Yang JC, Wu YL, Schuler M et al (2015) Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-lung 3 and LUX-lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 16:141–151
Naumov GN, Nilsson MB, Cascone T, Briggs A, Straume O, Akslen LA, Lifshits E, Byers LA, Xu L, HK W, Jänne P, Kobayashi S, Halmos B, Tenen D, Tang XM, Engelman J, Yeap B, Folkman J, Johnson BE, Heymach JV (2009) Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res 15:3484–3494
Ichihara Y, Yamaji K (2009) Effect of light conditions on the resistance of current-year Fagus Crenata seedlings against fungal pathogens causing damping-off in a natural beech forest: fungus isolation and histological and chemical resistance. J Chem Ecol 35:1077–1085
Gerlinger M, Rowan AJ, Horswell S et al (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366:883–892
Chen ZY, Zhong WZ, Zhang XC et al (2012) EGFR mutation heterogeneity and the mixed response to EGFR tyrosine kinase inhibitors of lung adenocarcinomas. Oncologist 17:978–985
Wu YL, Lee JS, Thongprasert S et al (2013) Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol 14:777–786
Giaccone G, Herbst RS, Manegold C, Scagliotti G, Rosell R, Miller V, Natale RB, Schiller JH, Von Pawel J, Pluzanska A, Gatzemeier U, Grous J, Ochs JS, Averbuch SD, Wolf MK, Rennie P, Fandi A, Johnson DH (2004) Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol 22:777–784
Herbst RS, Giaccone G, Schiller JH, Natale RB, Miller V, Manegold C, Scagliotti G, Rosell R, Oliff I, Reeves JA, Wolf MK, Krebs AD, Averbuch SD, Ochs JS, Grous J, Fandi A, Johnson DH (2004) Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol 22:785–794
Herbst RS, Prager D, Hermann R, Fehrenbacher L, Johnson BE, Sandler A, Kris MG, Tran HT, Klein P, Li X, Ramies D, Johnson DH, Miller VA, TRIBUTE Investigator Group (2005) TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 23:5892–5899
Acknowledgments
We thank the patients, their families, and all the investigators who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Supplementary Figure 1
(JPEG 54 kb)
Supplementary Figure 2
(JPEG 51 kb)
Rights and permissions
About this article
Cite this article
Tamiya, M., Tamiya, A., Shiroyama, T. et al. Phase1 study of cisplatin plus pemetrexed with erlotinib and bevacizumab for chemotherapy-naïve advanced non-squamous non-small cell lung cancer with EGFR mutations. Invest New Drugs 36, 608–614 (2018). https://doi.org/10.1007/s10637-017-0527-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0527-z