Summary
Background A single center phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab (GNP) to evaluate the safety and efficacy in metastatic pancreatic adenocarcinoma (PDAC) was conducted (NCT02331251). Methods PDAC patients (pts) with measurable disease, biopsy proven metastasis, adequate laboratory tests, and KPS ≥ 70% received GNP until progression or toxicity. Safety monitoring, RECIST 1.1, and irRECIST assessments were conducted. Response imaging was performed prior to cycle 4, then every 3 months. Changes in tumor cell-free DNA copy number instability (CNI) was retrospectively evaluated. Results 17 pts. with a median age of 56 were treated. 11 were women and all had a KPS of at least 80%. Grade 3 events occurred in 53% of patients. The phase II portion was completed for chemotherapy naïve PDAC pts. Of the 11 evaluable chemotherapy naïve PDAC, the disease control rate (partial response [PR] + stable disease[SD]) was 100%. There were 3 with PR on treatment for 8+, ~11, and 15 months; respectively. The primary endpoint of >15% complete response was not met. The median progression-free survival (PFS) and overall survival (OS) was 9.1 and 15.0 months for chemotherapy naïve treated patients. Of 9 patients evaluable for CNI change, a greater reduction in CNI correlated with longer PFS and improved OS. Conclusions GNP can be safely given to chemotherapy naïve PDAC patients. Efficacy appears to be slightly improved over previously reported results for standard weekly × 3 every 28 day gemcitabine and nab-paclitaxel dosing. CNI change may be prognostic for OS.


Similar content being viewed by others
Change history
24 April 2019
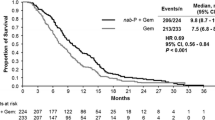
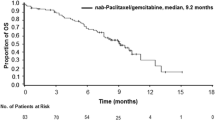
The authors would like to note an error in Figures��1 and 2 of this paper. The graph in Figure 1 incorrectly reflected the overall survival (OS), when it should have displayed the progression-free survival (PFS). The caption and median PFS values were correct.
24 April 2019
The authors would like to note an error in Figures��1 and 2 of this paper. The graph in Figure 1 incorrectly reflected the overall survival (OS), when it should have displayed the progression-free survival (PFS). The caption and median PFS values were correct.
Abbreviations
- AEs:
-
Adverse events
- CNI:
-
Tumor cell-free DNA copy number instability
- CI:
-
Confidence interval
- DC:
-
Disease control
- DLT:
-
Dose-limiting toxicity
- irAEs:
-
Immune-related adverse events
- irRECIST:
-
Immune-related response criteria
- GNP:
-
Gemcitabine and nab-paclitaxel
- MTD:
-
Maximum tolerated dose
- NSCLC:
-
Non-small cell lung cancer
- OS:
-
Overall survival
- PD:
-
Disease progression
- PDAC:
-
Pancreatic adenocarcinoma
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
PD-1 ligand
- PFS:
-
Progression-free survival
- RP2D:
-
Recommended phase 2 dose
- SD:
-
Stable disease
- TEAE:
-
Treatment-emergent AE
- WIRB:
-
Western Institutional Review Board
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30. https://doi.org/10.3322/caac.21387
Burris HA, Moore MJ, Andersen J et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413. https://doi.org/10.1200/JCO.1997.15.6.2403
Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825. https://doi.org/10.1056/NEJMoa1011923
Von Hoff DD, Ervin T, Arena FP et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703. https://doi.org/10.1056/NEJMoa1304369
Langer CJ, Gadgeel SM, Borghaei H et al (2016) Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 17:1497–1508. https://doi.org/10.1016/S1470-2045(16)30498-3
Weiss GJ, Waypa J, Blaydorn L et al (2017) A phase Ib study of pembrolizumab plus chemotherapy in patients with advanced cancer (PembroPlus). Br J Cancer 117:33–40. https://doi.org/10.1038/bjc.2017.145
Nishino M, Giobbie-Hurder A, Gargano M et al (2013) Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res 19:3936–3943. https://doi.org/10.1158/1078-0432.CCR-13-0895
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Weiss GJ, Beck J, Braun DP et al (2017) Tumor cell-free DNA copy number instability predicts therapeutic response to immunotherapy. Clin Cancer Res clincanres.0231.2017. https://doi.org/10.1158/1078-0432.CCR-17-0231
Masucci GV, Cesano A, Hawtin R et al (2016) Validation of biomarkers to predict response to immunotherapy in cancer: volume I - pre-analytical and analytical validation. J Immunother cancer 4:76. https://doi.org/10.1186/s40425-016-0178-1
Le DT, Durham JN, Smith KN et al (2017) Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357:409–413. https://doi.org/10.1126/science.aan6733
Skelton RA, Javed A, Zheng L, He J (2017) Overcoming the resistance of pancreatic cancer to immune checkpoint inhibitors. J Surg Oncol 116:55–62. https://doi.org/10.1002/jso.24642
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454. https://doi.org/10.1056/NEJMoa1200690
Le DT, Uram JN, Wang H et al (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509–2520. https://doi.org/10.1056/NEJMoa1500596
Wolchok JD, Kluger H, Callahan MK et al (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369:122–133. https://doi.org/10.1056/NEJMoa1302369
Duffy AG, Greten TF (2014) Immunological off-target effects of standard treatments in gastrointestinal cancers. Ann Oncol 25:24–32. https://doi.org/10.1093/annonc/mdt349
Galluzzi L, Senovilla L, Zitvogel L, Kroemer G (2012) The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 11:215–233. https://doi.org/10.1038/nrd3626
Jameson GS, Borazanci EH, Babiker HM et al (2017) A phase Ib/II pilot trial with nab-paclitaxel plus gemcitabine plus cisplatin in patients (pts) with stage IV pancreatic cancer. J Clin Oncol 35:341–341. https://doi.org/10.1200/JCO.2017.35.4_suppl.341
Wainberg ZA, Hochster HS, George B et al (2017) Phase I study of nivolumab (nivo) + nab -paclitaxel ( nab -P) ± gemcitabine (gem) in solid tumors: interim results from the pancreatic cancer (PC) cohorts. J Clin Oncol 35:412–412. https://doi.org/10.1200/JCO.2017.35.4_suppl.412
Johansson H, Andersson R, Bauden M et al (2016) Immune checkpoint therapy for pancreatic cancer. World J Gastroenterol 22:9457–9476. https://doi.org/10.3748/wjg.v22.i43.9457
Ahn DH, Krishna K, Blazer M et al (2017) A modified regimen of biweekly gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer is both tolerable and effective: a retrospective analysis. Ther Adv Med Oncol 9:75–82. https://doi.org/10.1177/1758834016676011
Acknowledgements
The authors express their gratitude and appreciation to all those that participated.
Funding
This study was funded by Western Regional Medical Center, Inc.
Author information
Authors and Affiliations
Contributions
Conception and design: G. Weiss, V. Khemka
Acquisition of data: All authors
Analysis and interpretation of data: All authors
Writing, review, and/or revision of the manuscript: All authors
Administrative, technical, or material support (eg, reporting or organizing data, constructing databases): All authors
Study supervision: G. Weiss, V. Khemka
Corresponding author
Ethics declarations
Conflict of interest
G. Weiss has been a paid consultant for Blend Therapeutics, Pharmatech, IDEA Pharma, AZ Medical Board, GLG Council, Ignyta, Circulogene Theranostics, Viomics, and Paradigm, has received speaker honorarium from Medscape, Merck, Novartis, and Pfizer; holds ownership interest in Circulogene Theranostics, and travel/accommodations from NantWorks, Cambridge Healthtech Institute, and Tesaro. J. Beck, K. Bornemann-Kolatzki, H. Urnovitz, and E. Schütz are employees of and hold ownership interest in Chronix Biomedical. V. Khemka has been a paid consultant for Axcess Oncology. No potential conflicts of interest were disclosed by the other authors.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Weiss, G.J., Blaydorn, L., Beck, J. et al. Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs 36, 96–102 (2018). https://doi.org/10.1007/s10637-017-0525-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0525-1