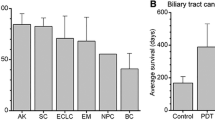
Summary
Photodynamic therapy (PDT) has drawn considerable attention for its efficacy against certain types of cancers. It shows however limits in the case of deep cancers, favoring tumor recurrence under suboptimal conditions. More insight into the molecular mechanisms of PDT-induced cytotoxicity and cytoprotection is essential to extend and strengthen this therapeutic modality. As PDT induces iNOS/NO in both tumor and microenvironment, we examined the role of nitric oxide (NO) in cytotoxicity and cytoprotection. Our findings show that NO mediates its cellular effects by acting on the NF-κB/YY1/RKIP loop, which controls cell growth and apoptosis. The cytoprotective effect of PDT-induced NO is observed at low NO levels, which activate the pro-survival/anti-apoptotic NF-κB and YY1, while inhibiting the anti-survival/pro-apoptotic and metastasis suppressor RKIP. In contrast, high PDT-induced NO levels inhibit NF-κB and YY1 and induce RKIP, resulting in significant anti-tumor activity. These findings reveal a critical role played by NO in PDT and suggest that the use of bifunctional PDT agents composed of a photosensitizer and a NO-donor could enhance the photo-treatment effect. A successful application of NO in anticancer therapy requires control of its concentration in the target tissue. To address this issue we propose as PDT agent, a bimolecular conjugate called DR2, composed of a photosensitizer (Pheophorbide a) and a non-steroidal anti-androgen molecule capable of releasing NO under the exclusive control of light. The mechanism of action of DR2 in prostate cancer cells is reported and discussed.








Similar content being viewed by others
References
Dougherty TJ (2002) An update on photodynamic therapy applications. J Clin Laser Med Surg 20:3–7
Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J (2011) Photodynamic therapy of cancer: an update. CA Cancer J Clin 61:250–281
Ferrario A, Gomer CJ (2010) Targeting the tumour microenvironment using photodynamic therapy combined with inhibitors of cyclooxygenase-2 or vascular endothelial growth factor. Methods Mol Biol 635:121–132
Dewaele M, Martinet W, Rubio N, Verfaillie T, de Witte PA, Piette J, Agostinis P (2011) Autophagy pathways activated in response to PDT contribute to cell resistance against ROS damage. J Cell Mol Med 15:1402–1414
Piette J (2015) Signalling pathway activation by photodynamic therapy: NF-κB at the crossroad between oncology and immunology. Photochem Photobiol Sci 14:1510–1517
Della Pietra E, Simonella F, Bonavida B, Xodo LE, Rapozzi V (2015) Repeated sub-optimal photodynamic treatments with pheophorbide a induce an epithelial mesenchymal transition in prostate cancer cells via nitric oxide. Nitric Oxide 45:43–53
Liang WM, Theng TSC, Lim KS, Tan WP (2014) Rapid development of squamous cell carcinoma after photodynamic therapy. Dermatol Surg 40:586–588
Gilaberte Y, Milla L, Salazar N, Vera-Alvarez J, Kourani O, Damian A, Rivarola V, Roca MJ, Espada J, González S, Juarranz A (2014) Cellular intrinsic factors involved in the resistance of squamous cell carcinoma to photodynamic therapy. J Invest Dermatol 134:2428–2437
Gomer CJ (2012) Induction of prosurvival molecules during treatment rethinking therapy options for photodynamic therapy. J Natl Compr Cancer Netw 10:S35–S39
Broekgaarden M, Weijer R, van Gulik TM, Hamblin MR, Heger M (2015) Tumour cell survival pathways activated by photodynamic therapy: a molecular basis for pharmacological inhibition strategies. Cancer Metastasis Rev 34:643–690
Gomer CJ, Ferrario A, Luna M, Rucker N, Wong S (2006) Photodynamic therapy: combined modality approaches targeting the tumour microenvironment. Lasers Surg Med 38:516–521
Wu K, Bonavida B (2009) The activated NF-kappaB-Snail-RKIP circuitry in cancer regulates both the metastatic cascade and resistance to apoptosis by cytotoxic drugs. Crit Rev Immunol 29:241–254
Bonavida B, Baritaki S (2011) Dual role of NO-donors in the reversal of tumour cell resistance and EMT: downregulation of the NF-kB/Snail/YY1/RKIP circuitry. Nitric Oxide 24:1–7
Gupta S, Ahmad N, Mukhtar H (1998) Involvement of nitric oxide during phthalocyanine (Pc4) photodynamic therapy-mediated apoptosis. Cancer Res 58:1785–1788
Rapozzi V, Umezawa K, Xodo LE (2011) Role of NF-κB/Snail/RKIP loop in the response of tumour cells to photodynamic therapy. Lasers Surg Med 43:575–585
Rapozzi V, Della Pietra E, Zorzet S, Zacchigna M, Bonavida B, Xodo LE (2013) Nitric oxide-mediated activity in anti-cancer photodynamic therapy. Nitric Oxide 30:26–35
Shan SQ, Rosner GL, Braun RD, Hahn J, Pearce C, Dewhirst MW (1997) Effects of diethylamine/nitric oxide on blood perfusion and oxygenation in the R3230Ac mammary carcinoma. Br J Cancer 76:429–437
Rapozzi V, Ragno D, Guerrini A, Ferroni C, Della Pietra E, Cesselli D, Castoria G, Di Donato M, Saracino E, Benfenati V, Varchi G (2015) Androgen receptor targeted conjugate for bimodal photodynamic therapy of prostate cancer in vitro. Bioconjug Chem 26:1662–1671
Chaiswing L, Zhong W, Oberley TD (2011) Distinct redox profiles of selected human prostate carcinoma cell lines: implications for rational design of redox therapy. Cancers (Basel) 3:3557–3584
Tai S, Sun Y, Squires JM, Zhang H, Oh WK, Liang CZ, Huang J (2011) PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 71:1668–1679
Azzouzi AR, Barret E, Moore CM, Villers A, Allen C, Scherz A, Muir G, de Wildt M, Barber NJ, Lebdai S, Emberton M (2013) TOOKAD(®) soluble vascular-targeted photodynamic (VTP) therapy: determination of optimal treatment conditions and assessment of effects in patients with localised prostate cancer. BJU Int 112:766–774
Davies KM, Wink DA, Saavedra JE, Keefer LK (2001) Chemistry of the diazeniumdiolati 2. Kinetics and mechanism of dissociation to nitric oxide in acqueous solution. J Am Chem Soc 123:5473–5481
Zuluaga MF, Lange N (2008) Combination of photodynamic therapy with anti-cancer agents. Curr Med Chem 15:1655–1673
Chen S, Cheng AC, Wang MS, Peng X (2008) Detection of apoptosis induced by new type gosling viral enteritis virus in vitro through fluorescein annexin V-FITC/PI double labeling. World J Gastroenterol 14:2174–2178
Yoo JO, Lim YC, Kim YM, Ha KS (2011) Differential cytotoxic responses to low- and high-dose photodynamic therapy in human gastric and bladder cancer cells. J Cell Biochem 112:3061–3071
Casas A, Di Venosa G, Hasan T, Batlle A (2011) Mechanisms of resistanceto photodynamic therapy. Curr Med Chem 18:2486–2515
Robey RW, Steadman K, Polgar O, Bates SE (2005) ABCG2-mediated transport of photosensitizer: potential impact on photodynamic therapy. Cancer Biol Ther 4:187–194
Selbo PK, Weyergang A, Eng MS, Bostad M, Maelandsmo GM, Hogset A, Berg K (2012) Strongly amphiphilic photosensitizers are not substrates of the cancer stem cell marker ABCG2 and provide specific and efficient light-triggered drug delivery of an EGFR-targeted cytotoxic drug. J Control Release 159:197–203
Singh A, Settleman J (2010) EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29:4741–4751
Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, CC W, LeBleu VS, Kalluri R (2015) Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527:525–530
Sanchez-Tillo E, Liu Y, de Barrios O, Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A, Postigo A (2012) EMT-activating transcription factors in cancer: beyond EMT and tumour invasiveness. Cell Mol Life Sci 69:3429–3456
Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 7:131–142
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA (2009) Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 119:1438–1449
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119:1420–1428
Thompson EW, Torri J, Sabol M, Sommers CL, Byers S, Valverius EM, Martin GR, Lippman ME, Stampfer MR, Dickson RB (1994) Oncogene-induced basement membrane invasiveness in human mammary epithelial cells. Clin Exp Metastasis 12:181–194
Boyer B, Vallés AM, Edme N (2000) Induction and regulation of epithelial-mesenchymal transitions. Biochem Pharmacol 60:1091–1099
Guarino M (2007) Epithelial-mesenchymal transition and tumour invasion. Int J Biochem Cell Biol 39:2153–2160
Yang J, Weinberg RA (2008) Epithelial mesenchymal transition: at the crossroads of development and tumour metastasis. Dev Cell 14:818–829
Zeisberg M, Neilson EG (2009) Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 119:1429–1437
Hay ED, Zuk A (1995) Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. Am J Kidney Dis 26:678–690
Vleminckx K, Vakaet L Jr, Mareel M, Fiers W, van Roy F (1991) Genetic manipulation of E-cadherin expression by epithelial tumour cells reveals an invasion suppressor role. Cell 66:107–119
Huber MA, Kraut N, Beug H (2005) Molecular requirements for epithelial-mesenchymal transition during tumour progression. Curr Opin Cell Biol 17:548–558
Boyer B, Tucker GC, Valles AM, Gavrilovic J, Thiery JP (1989) Reversible transition towards a fibroblastic phenotype in a rat carcinoma cell line. Int J Cancer Suppl 4:69–75
Raymond WA, Leong AS (1989) Vimentin-a new prognostic parameter in breast carcinoma? J Pathol 158:107–114
Carneiro ZA, de Moraes JC, Rodrigues FP, de Lima RG, Curti C, da Rocha ZN, Paulo M, Bendhack LM, Tedesco AC, Formiga AL, da Silva RS (2011) Photocytotoxic activity of a nitrosyl phthalocyanine ruthenium complex a system capable of producing nitric oxide and singlet oxygen. J Inorg Biochem 105:1035–1043
Acknowledgments
The authors are grateful to Dr. Derek Jones, for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Valentina Rapozzi declares that she has no conflict of interest. Greta Varchi declares that she has no conflict of interest. Emilia Della Pietra declares that she has no conflict of interest. Claudia Ferroni declares that she has no conflict of interest. Luigi Emilio Xodo declares that he has no conflict of interest.
Funding
This work was supported by personal research funds of the Dipartimento di Scienze Mediche e Biologiche (Department of Medical and Biological Sciences), University of Udine, Italy.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Electronic supplementary material
ESM 1
(DOCX 5400 kb)
Rights and permissions
About this article
Cite this article
Rapozzi, V., Varchi, G., Della Pietra, E. et al. A photodynamic bifunctional conjugate for prostate cancer: an in vitro mechanistic study. Invest New Drugs 35, 115–123 (2017). https://doi.org/10.1007/s10637-016-0396-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-016-0396-x