Summary
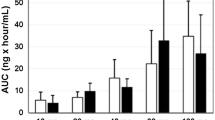
The primary objective of this phase I study of LY2780301, a dual p70 S6 kinase and Akt inhibitor, was to determine the recommended phase II dose as a single agent in patients with advanced cancer. Secondary objectives included safety, pharmacokinetic, and pharmacodynamic analyses, and co-clinical analyses in Avatar models. Eligible patients received total daily doses of LY2780301 100–500 mg, given orally as a single dose or divided into 2 doses for 28-day cycles. Dose escalation followed 3 + 3 design. The primary pharmacodynamic endpoint was inhibition of S6 assessed by skin and tumor biopsy. Thirty-two patients were treated. Common toxicities possibly related to treatment included constipation (19 %), fatigue (13 %), nausea (9 %), and diarrhea (9 %). Grade 3/4 toxicities potentially related to treatment were anemia (n = 2), increased alanine aminotransferase/aspartate aminotransferase (ALT) (n = 1), and increased gamma-glutamyl transpeptidase (GGT) (n = 1). One patient experienced best overall response of prolonged stable disease for 6 cycles. Plasma exposures of LY2780301 exceeded predicted efficacious exposures, but were not dose proportional. Among patients receiving 500 mg daily >50 % exhibited reduced S6 in skin biopsies at Day 8 of treatment, but the effect was not maintained. Plasma concentrations of LY2780301 and/or its metabolites were not correlated with S6 expression in the epidermis. There was minimal antitumor activity against the model, CRC 019. Avatar models showed minimal pharmacodynamic effects consistent with the observed antitumor effects. This study suggests a dose of LY2780301 500 mg QD for future studies.



Similar content being viewed by others
References
Takeuchi CS, Kim BG, Blazey CM MAS, Johnson HWB, Anand NK, Arcalas A, Baik TG, Buhr CA, Cannoy J, Epshteyn S, Joshi A, Lara K, Lee MS, Wang L, Leahy JW, Nuss JM, Aay N, Aoyama R, Foster P, Lee J, Lehoux I, Munagala N, Plonowski A, Rajan S, Woolfrey J, Yamaguchi K, Lamb P, Miller N (2013) Discovery of a novel class of highly potent, selective, ATP-competitive, and orally bioavailable inhibitors of the mammalian target of rapamycin (mTOR). J Med Chem 56:2218–2234
Zhou H, Luo Y, Huang S (2010) Updates of mTOR inhibitors. Anticancer Agents Med Chem 10:571–581
Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB (2005) Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov 4:988–1004
Rosen N, She QB (2006) AKT and cancer–is it all mTOR? Cancer Cell 10:254–256
Shaw RJ, Cantley LC (2006) Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441:424–430
Bartholomeusz C, Gonzales-Angulo AM (2012) Targeting the Pi3K signaling pathway in cancer therapy. Expert Opin Ther Targets 16:121–130
Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N (2001) Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev 15:2203–2208
Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ (2001) Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem 276:38349–38352
Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum MJ (2005) Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol 25:1869–1878
Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish JD, Coleman KG (2003) Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest 112:197–208
Gonzalez E, McGraw TE (2009) The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle 8:2502–2508
Tschopp O, Yang ZZ, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, Michaelis T, Frahm J, Hemmings BA (2005) Essential role of protein kinase B gamma (PKB gamma/Akt3) in postnatal brain development but not in glucose homeostasis. Development 132:2943–2954
Vivanco I, Sawyers C (2002) The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev Cancer 2:489–501
Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL, Zhang H, Soon-Shiong P, Shi T, Rajeshkumar NV, Maitra A, Hidalgo M (2011) Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 29:4548–4554
World Medical Assembly. Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Available at: www.wma.net/en/30publications/10policies/b3/. Accessed 9 February 2015
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. International Conference on Harmonization (ICH) Guideline for Good Clinical Practices- E6(R1) (1996). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdf. Accessed 9 February 2015
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
U.S. Department of Health and Human Services National Institutes of Health National Cancer Institute (2009) Common Terminology Criteria for Adverse Events (CTCAE) Version 4.02 NIH Publication No. 03–5410. http://evs.nci.nih.gov/ftp1/CTCAE/Archive/CTCAE_4.02_2009-09-15_QuickReference_8.5x11pdf 15. Accessed 9 February 2015
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V, International Harmonization Project on Lymphoma (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586
Chen Z, Cheng K, Walton Z, Wang Y, Ebi H, Shimamura T, Liu Y, Tupper T, Ouyang J, Li J, Gao P, Woo MS, Xu C, Yanagita M, Altabef A, Wang S, Lee C, Nakada Y, Peña CG, Sun Y, Franchetti Y, Yao C, Saur A, Cameron MD, Nishino M, Hayes DN, Wilkerson MD, Roberts PJ, Lee CB, Bardeesy N, Butaney M, Chirieac LR, Costa DB, Jackman D, Sharpless NE, Castrillon DH, Demetri GD, Jänne PA, Pandolfi PP, Cantley LC, Kung AL, Engelman JA, Wong KK (2012) A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature 483:613–617
Garrido-Laguna I, Tan AC, Uson M, Angenendt M, Ma WW, Villaroel MC, Zhao M, Rajeshkumar NV, Jimeno A, Donehower R, Iacobuzio-Donahue C, Barrett M, Rudek MA, Rubio-Viqueira B, Laheru D, Hidalgo M (2010) Integrated preclinical and clinical development of mTOR inhibitors in pancreatic cancer. Br J Cancer 103:649–655
Nardella C, Lunardi A, Patnaik A, Cantley LC, Pandolfi PP (2011) The APL paradigm and the “co-clinical trial” project. Cancer Discov 1:108–116
Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, Corà D, Di Nicolantonio F, Buscarino M, Petti C, Ribero D, Russolillo N, Muratore A, Massucco P, Pisacane A, Molinaro L, Valtorta E, Sartore-Bianchi A, Risio M, Capussotti L, Gambacorta M, Siena S, Medico E, Sapino A, Marsoni S, Comoglio PM, Bardelli A, Trusolino L (2011) A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov 1:508–523
Rubio-Viqueira B, Jimeno A, Cusatis G, Zhang X, Iacobuzio-Donahue C, Karikari C, Shi C, Danenberg K, Danenberg PV, Kuramochi H, Tanaka K, Singh S, Salimi-Moosavi H, Bouraoud N, Amador ML, Altiok S, Kulesza P, Yeo C, Messersmith W, Eshleman J, Hruban RH, Maitra A, Hidalgo M (2006) An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res 12:4652–4661
Del Bufalo D, Ciuffreda L, Trisciuoglio D, Desideri M, Cognetti F, Zupi G, Milella M (2006) Antiangiogenic potential of the Mammalian target of rapamycin inhibitor temsirolimus. Cancer Res 66:5549–5554
Dudkin L, Dilling MB, Cheshire PJ, Harwood FC, Hollingshead M, Arbuck SG, Travis R, Sausville EA, Houghton PJ (2001) Biochemical correlates of mTOR inhibition by the rapamycin ester CCI-779 and tumor growth inhibition. Clin Cancer Res 7:1758–1764
Geoerger B, Kerr K, Tang CB, Fung KM, Powell B, Sutton LN, Phillips PC, Janss AJ (2001) Antitumor activity of the rapamycin analog CCI-779 in human primitive neuroectodermal tumor/medulloblastoma models as single agent and in combination chemotherapy. Cancer Res 61:1527–1532
Afinitor®: East Hanover, NJ. Novartis Pharmaceuticals Corp. 2012
Squillace RM, Miller D, Cookson M, Wardwell SD, Moran L, Clapham D, Wang F, Clackson T, Rivera VM (2011) Antitumor activity of ridaforolimus and potential cell-cycle determinants of sensitivity in sarcoma and endometrial cancer models. Mol Cancer Ther 10:1959–1968
Juric D, Rodon J, Gonzalez-Angulo A, Burris HA, Bendell J, Berlin JD, Middleton MR, Bootle D, Boehm M, Schmitt A, Rouyrre N, Quadt C, Baselga J (2012) BYL719, a next generation PI3K alpha specific inhibitor: Preliminary safety, PK, and efficacy results from the first-in-human study. Cancer Res 72 (Suppl 1, abstract CT-01)
Ndubaku CO, Heffron TP, Staben ST, Baumgardner M, Blaquiere N, Bradley E, Bull R, Do S, Dotson J, Dudley D, Edgar KA, Friedman LS, Goldsmith R, Heald RA, Kolesnikov A, Lee L, Lewis C, Nannini M, Nonomiya J, Pang J, Price S, Prior WW, Salphati L, Sideris S, Wallin JJ, Wang L, Wei B, Sampath D, Olivero AG (2013) Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): a beta-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. J Med Chem 56:4597–4610
Davies BR, Greenwood H, Dudley P, Crafter C, Yu DH, Zhang J, Li J, Gao B, Ji Q, Maynard J, Ricketts SA, Cross D, Cosulich S, Chresta CC, Page K, Yates J, Lane C, Watson R, Luke R, Ogilvie D, Pass M (2012) Preclinical pharmacology of AZD5363, an inhibitor of AKT: pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther 11:873–887
Lin J, Sampath D, Nannini MA, Lee BB, Degtyarev M, Oeh J, Savage H, Guan Z, Hong R, Kassees R, Lee LB, Risom T, Gross S, Liederer BM, Koeppen H, Skelton NJ, Wallin JJ, Belvin M, Punnoose E, Friedman LS, Lin K (2013) Targeting activated Akt with GDC-0068, a novel selective Akt inhibitor that is efficacious in multiple tumor models. Clin Cancer Res 19:1760–1772
Sangai T, Akcakanat A, Chen H, Tarco E, Wu Y, Do KA, Miller TW, Arteaga CL, Mills GB, Gonzalez-Angulo AM, Meric-Bernstam F (2012) Biomarkers of response to Akt inhibitor MK-2206 in breast cancer. Clin Cancer Res 18:5816–28
She QB, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, DeFeo-Jones D, Huber HE, Rosen N (2008) Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS One 3, e3065
Yap TA, Walton MI, Hunter LJ, Valenti M, de Haven BA, Eve PD, Ruddle R, Heaton SP, Henley A, Pickard L, Vijayaraghavan G, Caldwell JJ, Thompson NT, Aherne W, Raynaud FI, Eccles SA, Workman P, Collins I, Garrett MD (2011) Preclinical pharmacology, antitumor activity, and development of pharmacodynamic markers for the novel, potent AKT inhibitor CCT128930. Mol Cancer Ther 10:360–371
Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, Baird RD, Delgado L, Taylor A, Lupinacci L, Riisnaes R, Pope LL, Heaton SP, Thomas G, Garrett MD, Sullivan DM, de Bono JS, Tolcher AW (2011) First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol 29:4688–4695
Elkabets M1, Vora S, Juric D, Morse N, Mino-Kenudson M, Muranen T, Tao J, Campos AB, Rodon J, Ibrahim YH, Serra V, Rodrik-Outmezguine V, Hazra S, Singh S, Kim P, Quadt C, Liu M, Huang A, Rosen N, Engelman JA, Scaltriti M, Baselga J (2013) mTORC1 Inhibition Is Required for Sensitivity to PI3K p110alpha Inhibitors in PIK3CA-Mutant Breast Cancer. Sci Transl Med 5: 196ra99
Acknowledgments
The study was sponsored by Eli Lilly and Company. The authors acknowledge Suzanne R.L. Young, Ph.D., and Jennifer M. Harris, Pharm.D., employees of Eli Lilly and Company, for writing assistance. Antonio Calles is a “Rio Hortega” fellowship grant recipient from the Instituto de Salud Carlos III (CM09/00283).
Conflicts of interest
A.A., J.R., A.C., I.B., P.P.L.C., and J.M. have no conflicts of interest to report. W.B. is an employee and shareholder of Onyx Pharmaceuticals, a subsidiary of Amgen, and is a shareholder of Eli Lilly and Company. M.H. received research support and an honorarium from Eli Lilly and Company. E.C. received clinical research support and an honorarium (Advisory Board) from Eli Lilly and Company. P.W., B.A.M., U.O., and K.A.B... are employees and shareholders of Eli Lilly and Company.
Previous disclosure
Data from this study, NCT01115751, were communicated as an oral presentation at The American Society of Clinical Oncology (ASCO) 2012 [J Clin Oncol 30, 2012 (suppl; abstr 3005).
Author information
Authors and Affiliations
Corresponding author
Additional information
At the time of this study, Dr. Miller and Mr. Bumgardner were employed by Eli Lilly and Company. Dr. Miller is currently employed by Everest Clinical Research, Little Falls, NJ, USA. Mr. Bumgardner is currently employed by Onyx Pharmaceuticals, a subsidiary of Amgen, South San Francisco, CA, USA.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 54 kb)
Rights and permissions
About this article
Cite this article
Azaro, A., Rodon, J., Calles, A. et al. A first-in-human phase I trial of LY2780301, a dual p70 S6 kinase and Akt Inhibitor, in patients with advanced or metastatic cancer. Invest New Drugs 33, 710–719 (2015). https://doi.org/10.1007/s10637-015-0241-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0241-7