Summary
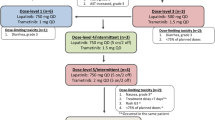
Purpose PI3K/AKT/mTOR and RAS/RAF/MEK pathways are frequently dysregulated in colorectal cancer (CRC). We conducted a biomarker-driven trial of the combination of MK-2206, an allosteric AKT 1/2/3 inhibitor, and selumetinib, a MEK 1/2 inhibitor, in patients with CRC to evaluate inhibition of phosphorylated ERK (pERK) and AKT (pAKT) in paired tumor biopsies. Patients and Methods Adult patients with advanced CRC were enrolled in successive cohorts stratified by KRAS mutation status. Initially, 12 patients received oral MK-2206 90 mg weekly with oral selumetinib 75 mg daily in 28-day cycles. Following an interim analysis, the doses of MK-2206 and selumetinib were increased to 135 mg weekly and 100 mg daily, respectively. Paired tumor biopsies were evaluated for target modulation. Results Common toxicities were gastrointestinal, hepatic, dermatologic, and hematologic. Of 21 patients enrolled, there were no objective responses. Target modulation did not achieve the pre-specified criteria of dual 70 % inhibition of pERK and pAKT levels in paired tumor biopsies. Conclusion Despite strong scientific rationale and preclinical data, clinical activity was not observed. The desired level of target inhibition was not achieved. Overlapping toxicities limited the ability to dose escalate to achieve exposures likely needed for clinical activity, highlighting the challenges in developing optimal combinations of targeted agents.





Similar content being viewed by others
References
Vaughn CP, Zobell SD, Furtado LV, Baker CL, Samowitz WS (2011) Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosomes Cancer 50(5):307–312. doi:10.1002/gcc.20854
Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F (2011) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29(15):2011–2019. doi:10.1200/JCO.2010.33.5091
Roy HK, Olusola BF, Clemens DL, Karolski WJ, Ratashak A, Lynch HT, Smyrk TC (2002) AKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesis. Carcinogenesis 23(1):201–205
Turke AB, Song Y, Costa C, Cook R, Arteaga CL, Asara JM, Engelman JA (2012) MEK inhibition leads to PI3K/AKT activation by relieving a negative feedback on ERBB receptors. Cancer Res 72(13):3228–3237. doi:10.1158/0008-5472.CAN-11-3747
Halilovic E, She QB, Ye Q, Pagliarini R, Sellers WR, Solit DB, Rosen N (2010) PIK3CA mutation uncouples tumor growth and cyclin D1 regulation from MEK/ERK and mutant KRAS signaling. Cancer Res 70(17):6804–6814. doi:10.1158/0008-5472.CAN-10-0409
Biondo A, Yap TA, Yan L, Patnaik A, Fearen I, Baird RD, Papadopoulos KP, Delgado LM, Taylor A, Lupinacci L, Blackman SC, Decordova S, Tall M, Heaton S, Garrett MD, Sullivan D, De Bono JS, Tolcher AW (2011) Phase I clinical trial of an allosteric AKT inhibitor, MK-2206, using a once weekly (QW) dose regimen in patients with advanced solid tumors. J Clin Oncol 29(suppl):3037, abstract
Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, Baird RD, Delgado L, Taylor A, Lupinacci L, Riisnaes R, Pope LL, Heaton SP, Thomas G, Garrett MD, Sullivan DM, de Bono JS, Tolcher AW (2011) First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol 29(35):4688–4695. doi:10.1200/JCO.2011.35.5263
Davies BR, Logie A, McKay JS, Martin P, Steele S, Jenkins R, Cockerill M, Cartlidge S, Smith PD (2007) AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther 6(8):2209–2219. doi:10.1158/1535-7163.MCT-07-0231
Tolcher AW, Khan K, Ong M, Banerji U, Papadimitrakopoulou V, Gandara DR, Patnaik A, Baird RD, Olmos D, Garrett CR, Skolnik JM, Rubin EH, Smith PD, Huang P, Learoyd M, Shannon KA, Morosky A, Tetteh E, Jou YM, Papadopoulos KP, Moreno V, Kaiser B, Yap TA, Yan L, de Bono JS (2014) Anti-tumour activity in RAS-driven tumours by blocking AKT and MEK. Clin Cancer Res. doi:10.1158/1078-0432.CCR-14-1901
Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, Kotani H (2010) MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther 9(7):1956–1967. doi:10.1158/1535-7163.MCT-09-1012
Rubinstein LV, Steinberg SM, Kummar S, Kinders R, Parchment RE, Murgo AJ, Tomaszewski JE, Doroshow JH (2010) The statistics of phase 0 trials. Stat Med 29(10):1072–1076. doi:10.1002/sim.3840
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. doi:10.1016/j.ejca.2008.10.026
Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, Hanson LJ, Gore L, Chow L, Leong S, Maloney L, Gordon G, Simmons H, Marlow A, Litwiler K, Brown S, Poch G, Kane K, Haney J, Eckhardt SG (2008) Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol 26(13):2139–2146. doi:10.1200/JCO.2007.14.4956
Banerji U, Camidge DR, Verheul HM, Agarwal R, Sarker D, Kaye SB, Desar IM, Timmer-Bonte JN, Eckhardt SG, Lewis KD, Brown KH, Cantarini MV, Morris C, George SM, Smith PD, van Herpen CM (2010) The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): a phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res 16(5):1613–1623. doi:10.1158/1078-0432.CCR-09-2483
Khan KH, Yan L, Mezynski J, Patnaik A, Moreno V, Papadopoulos KP, Garrett CR, Ong M, Shannon KA, Morosky A, Rubin EH, Tetteh E, Skolnik J, Smith IC, Smith PD, De Bono JS, Tolcher AW (2012) A phase I dose escalation study of oral MK-2206 (allosteric Akt inhibitor) with oral selumetinib (AZD6244; ARRY-142866) (MEK 1/2 inhibitor) in patients with advanced or metastatic solid tumors. J Clin Oncol 30(suppl):e13599, abstract
Watanabe T, Kobunai T, Yamamoto Y, Matsuda K, Ishihara S, Nozawa K, Iinuma H, Ikeuchi H, Eshima K (2011) Differential gene expression signatures between colorectal cancers with and without KRAS mutations: crosstalk between the KRAS pathway and other signalling pathways. Eur J Cancer 47(13):1946–1954. doi:10.1016/j.ejca.2011.03.029
Ang JE, Kaye S, Banerji U (2012) Tissue-based approaches to study pharmacodynamic endpoints in early phase oncology clinical trials. Curr Drug Targets 13(12):1525–1534
Acknowledgments
The authors thank the patients and their families for their contributions to this study. Additionally, we thank Dr. Andrea Regier Voth, Leidos Biomedical Research, Inc., for medical writing support, and Dr. Kate Ferry-Galow, Leidos Biomedical Research, Inc., for quality assurance auditing of the pharmacodynamic data presented in this manuscript.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contracts No. HHSN261200800001E and N01-CM-2011-00032. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. MK-2206 and selumetinib were generously provided by Merck and AstraZeneca, respectively, under a collaborative agreement with the Division of Cancer Treatment and Diagnosis, National Cancer Institute, NIH.
Conflict of interest
Michael H. Lam is an employee of Merck and Company. No potential conflicts of interest were disclosed by the other authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Khanh Do and Giovanna Speranza contributed equally to this work.
Rights and permissions
About this article
Cite this article
Do, K., Speranza, G., Bishop, R. et al. Biomarker-driven phase 2 study of MK-2206 and selumetinib (AZD6244, ARRY-142886) in patients with colorectal cancer. Invest New Drugs 33, 720–728 (2015). https://doi.org/10.1007/s10637-015-0212-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0212-z