Summary
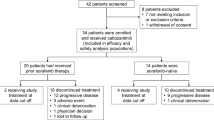
Background Several pilot studies have demonstrated the effectiveness of combination therapy with pyrimidine fluoride and interferon for advanced hepatocellular carcinoma.This study aimed to determine the recommended dose of capecitabine combined with peginterferon α-2a (Phase I) and evaluate its safety and efficacy for sorafenib-refractory advanced hepatocellular carcinoma (Phase II). Methods Capecitabine was administered daily on days 1–14, while peginterferon α-2a was administered on days 1, 8, and 15. The cycle was repeated every 21 days. The patients were scheduled to receive capecitabine [mg/(m2∙day)] and peginterferon α-2a (μg/week) at 3 dose levels in phase I: 1200 and 90 (level 1), 1600 and 90 (level 2), and 2000 and 90 (level 3), respectively. Results A total of 30 patients were enrolled. The recommended dose was level 3. Among the 24 patients receiving the drug at the recommended dosage, 2 (8 %) exhibited a partial response, 9 (38 %) exhibited stable disease, 10 (42 %) exhibited progressive disease, and 3 (13 %) were not evaluated. The median time to progression and overall survival were 3.0 months and 7.2 months, respectively. The most common toxicities were decreased white blood cell (88 %), neutrophil (88 %), and platelet counts (58 %); fatigue (50 %); and palmar–plantar erythrodysesthesia syndrome (42 %). Four patients (17 %) discontinued treatment because of severe adverse events. Conclusion Capecitabine at 2000 mg/(m2∙day) combined with peginterferon α-2a (90 μg/week) exhibited moderate, albeit manageable, toxicity and was declared as the recommended phase II dose. Further research is required to refine the efficacy of this combination.


Similar content being viewed by others
References
Wilhelm SM, Carter C, Tang L et al (2004) BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64:7099–7109
Llovet JM, Ricci S, Mazzaferro V (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Cheng AL, Kang YK, Chen Z et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10:25–34
Bruix J, Sherman M; American Association for the Study of Liver Diseases (2011) Management of hepatocellular carcinoma: an update. Hepatology 53:1020–1022
European Association for Study of Liver; European Organisation for Research and Treatment of Cancer (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–943
Sakon M, Nagano H, Dono K et al (2002) Combined intraarterial 5-fluorouracil and subcutaneous interferon-alpha therapy for advanced hepatocellular carcinoma with tumor thrombi in the major portal branches. Cancer 94:435–442
Obi S, Yoshida H, Toune R et al (2006) Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer 106:1990–1997
Ota H, Nagano H, Sakon M et al (2005) Treatment of hepatocellular carcinoma with major portal vein thrombosis by combined therapy with subcutaneous interferon-alpha and intra-arterial 5-fluorouracil; role of type 1 interferon receptor expression. Br J Cancer 93:557–564
Ueshima K, Kudo M, Nagai T et al (2008) Combination therapy with S-1 and pegylated interferon alpha for advanced hepatocellular carcinoma. Oncology 75:106–113
Uchino K, Obi S, Tateishi R et al (2012) Systemic combination therapy of intravenous continuous 5-fluorouracil and subcutaneous pegylated interferon alfa-2a for advanced hepatocellular carcinoma. J Gastroenterol 47:1152–1159
Twelves C, Glynne-Jones R, Cassidy J et al (1999) Effect of hepatic dysfunction due to liver metastases on the pharmacokinetics of capecitabine and its metabolites. Clin Cancer Res 5:1696–1702
Patt YZ, Hassan MM, Aguayo A et al (2004) Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer 101:578–586
von Delius S, Lersch C, Mayr M et al (2007) Capecitabine for treatment of advanced hepatocellular carcinoma. Hepatogastroenterology 54:2310–2314
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Yau T, Wong H, Chan P et al (2012) Phase II study of bevacizumab and erlotinib in the treatment of advanced hepatocellular carcinoma patients with sorafenib-refractory disease. Investig New Drugs 30:2384–2390
Finn RS, Kang YK, Mulcahy M et al (2012) Phase II, open-label study of brivanib as second-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res 18:2090–2098
Llovet JM, Decaens T, Raoul JL et al (2013) Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol 3:3509–3516
Bruix J, Tak WY, Gasbarrini A et al (2013) Regorafenib as second-line therapy for intermediate or advanced hepatocellular carcinoma: multicentre, open-label, phase II safety study. Eur J Cancer 49:3412–3419
Boige V, Raoul JL, Pignon JP et al (2007) Multicentre phase II trial of capecitabine plus oxaliplatin (XELOX) in patients with advanced hepatocellular carcinoma: FFCD 03-03 trial. Br J Cancer 97:862–867
Shim JH, Park JW, Nam BH et al (2009) Efficacy of combination chemotherapy with capecitabine plus cisplatin in patients with unresectable hepatocellular carcinoma. Cancer Chemother Pharmacol 63:459–467
Kondo M, Nagano H, Wada H et al (2005) Combination of IFN-alpha and 5-fluorouracil induces apoptosis through IFN-alpha/beta receptor in human hepatocellular carcinoma cells. Clin Cancer Res 11:1277–1286
Xiao YS, Tang ZY, Fan J et al (2004) Interferon-alpha 2a up-regulated thymidine phosphorylase and enhanced antitumor effect of capecitabine on hepatocellular carcinoma in nude mice. J Cancer Res Clin Oncol 30:546–550
Katsura Y, Wada H, Murakami M et al (2013) PTK787/ZK222584 combined with interferon alpha and 5-fluorouracil synergistically inhibits vegf signaling pathway in hepatocellular carcinoma. Ann Surg Oncol 20:517–526
Acknowledgments
We express our heartfelt thanks and gratitude to the patients and their families.
Conflicts of Interest
Prof. Osamu Yokosuka received research grants from Chugai Pharmaceutical Co., Ltd. Sadahisa Ogasawara, Tetsuhiro Chiba, Yoshihiko Ooka, Naoya Kanogawa, Tenyu Motoyama, Eiichiro Suzuki, Akinobu Tawada, and Fumihiko Kanai declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogasawara, S., Chiba, T., Ooka, Y. et al. A phase I/II trial of capecitabine combined with peginterferon α-2a in Patients with sorafenib-refractory advanced hepatocellular carcinoma. Invest New Drugs 32, 762–768 (2014). https://doi.org/10.1007/s10637-014-0097-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0097-2