Summary
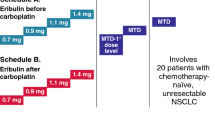
Background. A phase I, dose-escalation study of AT-101 with cisplatin and etoposide was conducted to determine the maximum tolerated dose (MTD)/recommended phase 2 dose (RP2D), safety and pharmacokinetics in patients with advanced solid tumors, with an expanded cohort in patients with extensive-stage small cell lung cancer (ES-SCLC) to assess preliminary activity. Methods. In the dose escalation portion, increasing doses of AT-101 were administered orally BID on days 1–3 along with cisplatin on day 1 and etoposide on days 1–3 of a 21 day cycle. At the RP2D, an additional 7 patients with untreated ES-SCLC were enrolled. Results. Twenty patients were enrolled in the dose-escalation cohort, and 7 patients with ES-SCLC were enrolled in the expanded cohort. The MTD/RP2D was established at AT-101 40 mg BID days 1–3 with cisplatin 60 mg/m2 and etoposide 120 mg/m2 on day 1 of a 21 day cycle with pegfilgrastim support. Two DLTs of neutropenic fever were seen at dose level 1. After the addition of pegfilgrastim, no additional DLTs were observed. Grade 3/4 treatment-related toxicities included: diarrhea, increased AST, neutropenia, hypophosphatemia, hyponatremia, myocardial infarction and pulmonary embolism. No apparent PK interactions were observed between the agents. Preliminary activity was observed with PRs in patients with ES-SCLC, high-grade neuroendocrine tumor, esophageal cancer and NSCLC. Conclusions. AT-101 with cisplatin and etoposide is well tolerated with growth factor support. Anti-tumor activity was observed in a variety of cancers including ES-SCLC, supporting further investigation with BH-3 mimetics in combination with standard chemotherapy for ES-SCLC.
Similar content being viewed by others
References
World Health Organization (2012) Cancer. Fact Sheet No 298. February 2012. Accessed on January 12, 2013
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA: A Cancer J Clin 62:10–29
van Meerbeeck JP, Fennell DA, De Ruysscher DKM (2011) Small-cell lung cancer. Lancet 378:1741–1755. doi:10.1016/S0140-6736(11)60165-7
Sundstrøm S, Bremnes RM, Kaasa S et al (2002) Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years’ follow-up. J Clin Oncol 20:4665–4672
Ikegaki N, Katsumata M, Minna J, Tsujimoto Y (1994) Expression of bcl-2 in small cell lung carcinoma cells. Cancer Res 54:6–8
Sartorius UA, Krammer PH (2002) Upregulation of Bcl-2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer 97:584–592
Adams JM, Cory S (1998) The Bcl-2 protein family: arbiters of cell survival. Science 281:1322–1326
Kroemer G (1997) The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med 3:614–620
Heiser D, Labi V, Erlacher M, Villunger A (2004) The Bcl-2 protein family and its role in the development of neoplastic disease. Exp Gerontol 39:1125–1135. doi:10.1016/j.exger.2004.04.011
Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619
Leri A, Claudio PP, Li Q et al (1998) Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J Clin Invest 101:1326–1342. doi:10.1172/JCI316
Tang XC, Zhu MK, Shi QX (1980) Comparative studies on the absorption, distribution and excretion of 14C-gossypol in four species of animals (author’s transl). Yao Xue Xue Bao 15:212–217
Zangemeister-Wittke U, Ziegler A (1998) Bcl-2 antisense therapy for cancer: the art of persuading tumour cells to commit suicide. Apoptosis 3:67–74
Wang G, Min P, Zhang Y, et al. (2007) Preclinical studies of orally active, pan Bcl-2 small molecule inhibitor AT-101 in small cell lung cancer. AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics Abstracts A:51
James DF, Castro JE, Loria O et al (2006) AT-101, a small molecule Bcl-2 antagonist, in treatment naive CLL patients (pts) with high risk features: preliminary results from an ongoing phase I trial. ASCO Meet Abstr 24:6605
Liu G, Kelly WK, Wilding G et al (2009) An open-label, multicenter, phase I/II study of single-agent AT-101 in men with castrate-resistant prostate cancer. Clin Cancer Res 15:3172–3176. doi:10.1158/1078-0432.CCR-08-2985
Fiveash JB, Chowdhary SA, Peereboom D et al (2009) NABTT-0702: A phase II study of R-(−)-gossypol (AT-101) in recurrent glioblastoma multiforme (GBM). ASCO Meet Abstr 27:2010
Saleh M, Pitot H, Hartung J, et al. (2005) Phase I trial of AT-101, an orally bioavailable inhibitor of Bcl-2, in patients with advanced malignancies. AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics Abstracts C:89.
Pitot HC, Saleh M, Holmlund J et al (2007) Extended phase I trial of the oral pan-Bcl-2 inhibitor AT-101 by multiple dosing schedules in patients with advanced cancers. ASCO Meet Abstr 25:3583
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216. doi:10.1093/jnci/92.3.205
Lopez-Flores A, Jurado R, Garcia-Lopez P (2005) A high-performance liquid chromatographic assay for determination of cisplatin in plasma, cancer cell, and tumor samples. J Pharmacol Toxicol Methods 52:366–372. doi:10.1016/j.vascn.2005.06.005
Shirazi FH, Bahrami G, Stewart DJ et al (2001) A rapid reversed phase high performance liquid chromatographic method for determination of etoposide (VP-16) in human plasma. J Pharm Biomed Anal 25:353–356
You B, Tranchand B, Girard P et al (2008) Etoposide pharmacokinetics and survival in patients with small cell lung cancer: a multicentre study. Lung Cancer 62:261–272. doi:10.1016/j.lungcan.2008.03.008
Verschraagen M, Boven E, Ruijter R et al (2003) Pharmacokinetics and preliminary clinical data of the novel chemoprotectant BNP7787 and cisplatin and their metabolites. Clin Pharmacol Ther 74:157–169. doi:10.1016/S0009-9236(03)00150-4
Baggstrom MQ, Qi Y, Koczywas M et al (2011) A phase II study of AT-101 (Gossypol) in chemotherapy-sensitive recurrent extensive-stage small cell lung cancer. J Thorac Oncol 6:1757–1760. doi:10.1097/JTO.0b013e31822e2941
Sonpavde G, Matveev V, Burke JM et al (2012) Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule Bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. Ann Oncol 23:1803–1808. doi:10.1093/annonc/mdr555
Heist RS, Fain J, Chinnasami B et al (2010) Phase I/II study of AT-101 with topotecan in relapsed and refractory small cell lung cancer. J Thorac Oncol 5:1637–1643. doi:10.1097/JTO.0b013e3181e8f4dc
Ready N, Karaseva NA, Orlov SV et al (2011) Double-blind, placebo-controlled, randomized phase 2 study of the proapoptotic agent AT-101 plus docetaxel, in second-line non-small cell lung cancer. J Thorac Oncol 6:781–785. doi:10.1097/JTO.0b013e31820a0ea6
Schiller JH, Adak S, Cella D et al (2001) Topotecan versus observation after cisplatin plus etoposide in extensive-stage small-cell lung cancer: E7593–a phase III trial of the Eastern Cooperative Oncology Group. J Clin Oncol 19:2114–2122
Hanna N, Bunn PA, Langer C et al (2006) Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol 24:2038–2043. doi:10.1200/JCO.2005.04.8595
Zatloukal P, Cardenal F, Szczesna A et al (2010) A multicenter international randomized phase III study comparing cisplatin in combination with irinotecan or etoposide in previously untreated small-cell lung cancer patients with extensive disease. Ann Oncol 21:1810–1816. doi:10.1093/annonc/mdq036
Coutinho EM (2002) Gossypol: a contraceptive for men. Contraception 65:259–263
Bunn PA, Carney DN (1997) Overview of chemotherapy for small cell lung cancer. Semin Oncol 24:S7–69–S7–74
Sandler AB (1997) Current management of small cell lung cancer. Semin Oncol 24:463–476
Acknowledgments
This study was supported by NCI and the following grants - NCI UO1 CA062491, SAIC 25XS097, and 1ULRR025011.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schelman, W.R., Mohammed, T.A., Traynor, A.M. et al. A phase I study of AT-101 with cisplatin and etoposide in patients with advanced solid tumors with an expanded cohort in extensive-stage small cell lung cancer. Invest New Drugs 32, 295–302 (2014). https://doi.org/10.1007/s10637-013-9999-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-013-9999-7