Summary
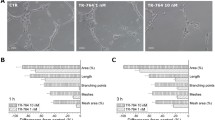
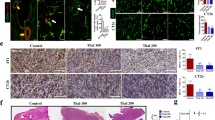
Vascular disrupting agents (VDAs) are new class of anti-cancer drugs targeting pre-existing tumor vasculature which lead to tumor ischemia and necrosis. An innovative tubulin polymerization inhibitor, CKD-516, was recently developed as a VDA. We attempted to evaluate its tubulin destabilizing effect using immunofluorescence staining on human endothelial cells (HUVECs) and to ascertain its antivascular effect in a rabbit VX2 tumor model using dynamic contrast-enhanced (DCE) MRI by measuring the changes in kinetic parameters such as K-trans and IAUGC. Immunofluorescence staining using anti-tubulin and anti-actin antibodies on HUVECs showed that CKD-516 selectively disrupted tubulin component of the endothelial cytoskeleton. Serial DCE-MRI showed a significant decrease in K-trans and IAUGC parameters from baseline at 4 h (39.9 % in K-trans; −45.0 % in IAUGC) and at 24 h (−32.2 % in K-trans; −36.5 % in IAUGC), and a significant recovery at 48 h (22.9 % in K-trans; 34.8 % in IAUGC) following administration of CKD-516 at a 0.7-mg/kg dose. When the tumors were stratified according to the initial K-trans value of 0.1, tumors with a high K-trans > 0.1 which was indicative of having well-developed pre-existing vessels, showed greater reduction in K-trans and IAUGC values. On histologic examination, the degree of necrosis of treated tumors was significantly greater than that of untreated tumors. In summary, CKD-516 is an effective VDA which results in rapid vascular shutdown by targeting the tubulin component of tumor vessels and thus leads to necrosis.






Similar content being viewed by others
References
McKeage MJ, Baguley BC (2010) Disrupting established tumor blood vessels: an emerging therapeutic strategy for cancer. Cancer 116:1859–1871
Delmonte A, Sessa C (2009) Ave8062: a new combretastatin derivative vascular disrupting agent. Expert Opin Investig Drugs 18:1541–1548
Zweifel M, Padhani AR (2010) Perfusion mri in the early clinical development of antivascular drugs: decorations or decision making tools? Eur J Nucl Med Mol Imaging 37(Suppl 1):S164–S182
Wang H, Marchal G, Ni Y (2011) Multiparametric mri biomarkers for measuring vascular disrupting effect on cancer. World J Radiol 3:1–16
Wang H, Li J, Chen F, De Keyzer F, Yu J, Feng Y, Nuyts J, Marchal G, Ni Y (2010) Morphological, functional and metabolic imaging biomarkers: assessment of vascular-disrupting effect on rodent liver tumours. Eur Radiol 20:2013–2026
Lee J, Bae S, Lee SH, Choi H, Kim YH, Kim SJ, Park GT, Moon SK, Kim DH, Lee S, Ahn SK, Choi NS, Lee KJ (2010) Discovery of a potent tubulin polymerization inhibitor: synthesis and evaluation of water-soluble prodrugs of benzophenone analog. Bioorg Med Chem Lett 20:6327–6330
Lee J, Kim SJ, Choi H, Kim YH, Lim IT, Yang HM, Lee CS, Kang HR, Ahn SK, Moon SK, Kim DH, Lee S, Choi NS, Lee KJ (2010) Identification of ckd-516: a potent tubulin polymerization inhibitor with marked antitumor activity against murine and human solid tumors. J Med Chem 53:6337–6354
Lunt SJ, Akerman S, Hill SA, Fisher M, Wright VJ, Reyes-Aldasoro CC, Tozer GM, Kanthou C (2010) Vascular effects dominate solid tumor response to treatment with combretastatin a-4-phosphate. Int J Cancer
Kidd JG, Rous P (1940) A transplantable rabbit carcinoma originating in a virus-induced papilloma and containing the virus in masked or altered form. J Exp Med 71:813–838
Tofts PS, Kermode AG (1991) Measurement of the blood-brain barrier permeability and leakage space using dynamic mr imaging. 1. Fundamental concepts. Magn Reson Med 17:357–367
Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ, Port RE, Taylor J, Weisskoff RM (1999) Estimating kinetic parameters from dynamic contrast-enhanced t(1)-weighted mri of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging 10:223–232
Weidner N (1999) Tumour vascularity and proliferation: clear evidence of a close relationship. J Pathol 189:297–299
Jiang HJ, Zhang ZR, Shen BZ, Wan Y, Guo H, Li JP (2009) Quantification of angiogenesis by ct perfusion imaging in liver tumor of rabbit. Hepatobiliary Pancreat Dis Int 8:168–173
Deng ZT, Feng T, Wang P, Qi X, Chen XH, Li YX, Song CL, Geng MY, Li J (2011) Effects of the novel vascular targeting agent mds-11p on tumor vascularity and its antitumor activity. Biochem Pharmacol 82:1832–1842
Davis PD, Dougherty GJ, Blakey DC, Galbraith SM, Tozer GM, Holder AL, Naylor MA, Nolan J, Stratford MR, Chaplin DJ, Hill SA (2002) Zd6126: a novel vascular-targeting agent that causes selective destruction of tumor vasculature. Cancer Res 62:7247–7253
Lee TY, Gotlieb AI (2003) Microfilaments and microtubules maintain endothelial integrity. Microsc Res Tech 60:115–127
Siemann DW, Mercer E, Lepler S, Rojiani AM (2002) Vascular targeting agents enhance chemotherapeutic agent activities in solid tumor therapy. Int J Cancer 99:1–6
Mason RP, Zhao D, Liu L, Trawick ML, Pinney KG (2011) A perspective on vascular disrupting agents that interact with tubulin: preclinical tumor imaging and biological assessment. Integr Biol (Camb) 3:375–387
Gaya A, Daley F, Taylor NJ, Tozer G, Qureshi U, Padhani A, Pedley RB, Begent R, Wellsted D, Stirling JJ, Rustin G (2008) Relationship between human tumour angiogenic profile and combretastatin-induced vascular shutdown: an exploratory study. Br J Cancer 99:321–326
Lavisse S, Lejeune P, Rouffiac V, Elie N, Bribes E, Demers B, Vrignaud P, Bissery MC, Brule A, Koscielny S, Peronneau P, Lassau N (2008) Early quantitative evaluation of a tumor vasculature disruptive agent ave8062 using dynamic contrast-enhanced ultrasonography. Invest Radiol 43:100–111
Luo Y, Hradil VP, Frost DJ, Rosenberg SH, Gordon GB, Morgan SJ, Gagne GD, Cox BF, Tahir SK, Fox GB (2009) Abt-751, a novel tubulin-binding agent, decreases tumor perfusion and disrupts tumor vasculature. Anticancer Drugs 20:483–492
Wang H, Sun X, Chen F, De Keyzer F, Yu J, Landuyt W, Vandecaveye V, Peeters R, Bosmans H, Hermans R, Marchal G, Ni Y (2009) Treatment of rodent liver tumor with combretastatin a4 phosphate: noninvasive therapeutic evaluation using multiparametric magnetic resonance imaging in correlation with microangiography and histology. Invest Radiol 44:44–53
Vincent L, Kermani P, Young LM, Cheng J, Zhang F, Shido K, Lam G, Bompais-Vincent H, Zhu Z, Hicklin DJ, Bohlen P, Chaplin DJ, May C, Rafii S (2005) Combretastatin a4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signaling. J Clin Investig 115:2992–3006
Acknowledgements
This work was partially supported by a National Research Foundation of Korea (NRF) Grant funded by the Korean Government (2011-0026127) and by grants from the Korean Health and Medical Technology R&DProject, Ministry of Health, Welfare, and Family Affairs (A100895, A100265). We thank Bonnie Hami, M.A. (USA) for her editorial assistance. We also thank Hyunjun Ji and Peter Gall for his technical advice regarding Tissue 4D software.
Conflict of interest
One coauthors (Berthold Kiefer), an employee of Siemens Healthcare, provided technical advice for DCE-MRI in animal experiment and has no direct or indirect conflict interests. One coauthor (Chin Kim), an employee of Chong Kun Dang Pharmaceuticals, provided CKD-516 and technical advice for making drug formulation and in vitro experiment. The other coauthors (Kyung Won Kim, Jeong Min Lee, Yong Sik Jeon, In Joon Lee, YoonSeok Choi, Jisuk Park, Joon Koo Han, Byung Ihn Choi) have no direct or indirect conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, K.W., Lee, J.M., Jeon, Y.S. et al. Vascular disrupting effect of CKD-516: preclinical study using DCE-MRI. Invest New Drugs 31, 1097–1106 (2013). https://doi.org/10.1007/s10637-012-9915-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-012-9915-6