Summary
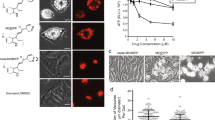
This study was designed to test the hypothesis that specific inhibition of cathepsins B and L will cause death of neuroblastoma cells. Five compounds that differ in mode and rate of inhibition of these two enzymes were all shown to cause neuroblastoma cell death. Efficacy of the different compounds was related to their ability to inhibit the activity of the isolated enzymes. A dose- and time-response for induction of cell death was demonstrated for each compound. A proteomic study showed that inhibitor treatment caused an increase of markers of cell stress, including induction of levels of the autophagy marker, LC-3-II. Levels of this marker protein were highest at cytotoxic inhibitor concentrations, implicating autophagy in the cell death process. An in vivo mouse model showed that one of these inhibitors markedly impaired tumor growth. It is concluded that development of drugs to target these two proteases may provide a novel approach to treating neuroblastoma.





Similar content being viewed by others
References
Maris JM (2010) Recent advances in neuroblastoma. N Engl J Med 362(23):2202–2211. doi:10.1056/NEJMra0804577
van Noesel MM, Versteeg R (2004) Pediatric neuroblastomas: genetic and epigenetic ‘danse macabre’. Gene 325:1–15
Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, Gerbing RB, Reynolds CP (1999) Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med 341(16):1165–1173. doi:10.1056/NEJM199910143411601
Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM (1984) Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 224(4653):1121–1124
Mosse YP, Laudenslager M, Longo L, Cole KA, Wood A, Attiyeh EF, Laquaglia MJ, Sennett R, Lynch JE, Perri P, Laureys G, Speleman F, Kim C, Hou C, Hakonarson H, Torkamani A, Schork NJ, Brodeur GM, Tonini GP, Rappaport E, Devoto M, Maris JM (2008) Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 455(7215):930–935. doi:10.1038/nature07261
Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, Shimada H, Grupp SA, Seeger R, Reynolds CP, Buxton A, Reisfeld RA, Gillies SD, Cohn SL, Maris JM, Sondel PM, Children’s Oncology G (2010) Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 363(14):1324–1334. doi:10.1056/NEJMoa0911123
Felbor U, Kessler B, Mothes W, Goebel HH, Ploegh HL, Bronson RT, Olsen BR (2002) Neuronal loss and brain atrophy in mice lacking cathepsins B and L. Proc Natl Acad Sci U S A 99(12):7883–7888. doi:10.1073/pnas.112632299
Ambroso JL, Harris C (1994) In vitro embryotoxicity of the cysteine proteinase inhibitors benzyloxycarbonyl-phenylalanine-alanine-diazomethane (Z-Phe-Ala-CHN2) and benzyloxycarbonyl-phenylalanine-phenylalanine-diazomethane (Z-Phe-Phe-CHN2). Teratology 50(3):214–228. doi:10.1002/tera.1420500307
Mason RW, Stabley DL, Picerno GN, Frenck J, Xing S, Bertenshaw GP, Sol-Church K (2002) Evolution of placental proteases. Biol Chem 383(7–8):1113–1118. doi:10.1515/BC.2002.120
Mason RW (2008) Emerging functions of placental cathepsins. Placenta 29(5):385–390. doi:10.1016/j.placenta.2008.02.006
Falgueyret JP, Desmarais S, Oballa R, Black WC, Cromlish W, Khougaz K, Lamontagne S, Masse F, Riendeau D, Toulmond S, Percival MD (2005) Lysosomotropism of basic cathepsin K inhibitors contributes to increased cellular potencies against off-target cathepsins and reduced functional selectivity. J Med Chem 48(24):7535–7543. doi:10.1021/jm0504961
Desmarais S, Black WC, Oballa R, Lamontagne S, Riendeau D, Tawa P, le Duong T, Pickarski M, Percival MD (2008) Effect of cathepsin k inhibitor basicity on in vivo off-target activities. Mol Pharmacol 73(1):147–156. doi:10.1124/mol.107.039511
Anagli J, Abounit K, Stemmer P, Han Y, Allred L, Weinsheimer S, Movsisyan A, Seyfried D (2008) Effects of cathepsins B and L inhibition on postischemic protein alterations in the brain. Biochem Biophys Res Commun 366(1):86–91. doi:10.1016/j.bbrc.2007.11.104
Rice D, Barone S Jr (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Heal Perspect 108(Suppl 3):511–533
Clancy B, Darlington RB, Finlay BL (2001) Translating developmental time across mammalian species. Neuroscience 105(1):7–17
Colella R, Lu G, Glazewski L, Korant B, Matlapudi A, England MR, Craft C, Frantz CN, Mason RW (2010) Induction of cell death in neuroblastoma by inhibition of cathepsins B and L. Cancer Lett 294(2):195–203. doi:10.1016/j.canlet.2010.01.037
Crawford C, Mason RW, Wikstrom P, Shaw E (1988) The design of peptidyldiazomethane inhibitors to distinguish between the cysteine proteinases calpain-Ii, cathepsin-L and cathepsin-B. Biochem J 253(3):751–758
Xing R, Mason RW (1998) Design of a transferrin-proteinase inhibitor conjugate to probe for active cysteine proteinases in endosomes. Biochem J 336(Pt 3):667–673
Hassanein M, Bojja AS, Glazewski L, Lu G, Mason RW (2009) Protein processing by the placental protease, cathepsin P. Mol Hum Reprod 15(7):433–442. doi:10.1093/molehr/gap029
Mason RW, Green GDJ, Barrett AJ (1985) Human-liver cathepsin-L. Biochem J 226(1):233–241
Biroc SL, Gay S, Hummel K, Magill C, Palmer JT, Spencer DR, Sa S, Klaus JL, Michel BA, Rasnick D, Gay RE (2001) Cysteine protease activity is up-regulated in inflamed ankle joints of rats with adjuvant-induced arthritis and decreases with in vivo administration of a vinyl sulfone cysteine protease inhibitor. Arthritis Rheum 44(3):703–711. doi:10.1002/1529-0131(200103)44:3<703::AID-ANR120>3.0.CO;2-2
Sajid M, Robertson SA, Brinen LS, McKerrow JH (2011) Cruzain: the path from target validation to the clinic. Adv Exp Med Biol 712:100–115. doi:10.1007/978-1-4419-8414-2_7
Behrends C, Sowa ME, Gygi SP, Harper JW (2010) Network organization of the human autophagy system. Nature 466(7302):68–76. doi:10.1038/nature09204
Palmer JT, Bryant C, Wang DX, Davis DE, Setti EL, Rydzewski RM, Venkatraman S, Tian ZQ, Burrill LC, Mendonca RV, Springman E, McCarter J, Chung T, Cheung H, Janc JW, McGrath M, Somoza JR, Enriquez P, Yu ZW, Strickley RM, Liu L, Venuti MC, Percival MD, Falgueyret JP, Prasit P, Oballa R, Riendeau D, Young RN, Wesolowski G, Rodan SB, Johnson C, Kimmel DB, Rodan G (2005) Design and synthesis of tri-ring P3 benzamide-containing aminonitriles as potent, selective, orally effective inhibitors of cathepsin K. J Med Chem 48(24):7520–7534. doi:10.1021/jm058198r
Xing R, Addington AK, Mason RW (1998) Quantification of cathepsins B and L in cells. Biochem J 332(Pt 2):499–505
Yorimitsu T, Klionsky DJ (2007) Eating the endoplasmic reticulum: quality control by autophagy. Trends Cell Biol 17(6):279–285
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19(21):5720–5728. doi:10.1093/emboj/19.21.5720
Boya P, Gonzalez-Polo RA, Poncet D, Andreau K, Vieira HL, Roumier T, Perfettini JL, Kroemer G (2003) Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene 22(25):3927–3936
Boya P, Kroemer G (2008) Lysosomal membrane permeabilization in cell death. Oncogene 27(50):6434–6451
Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A (2009) Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ 16(7):966–975. doi:10.1038/cdd.2009.33
Xing RY, Wu F, Mason RW (1998) Control of breast tumor cell growth using a targeted cysteine protease inhibitor. Cancer Res 58(5):904–909
Tu C, Ortega-Cava CF, Chen G, Fernandes ND, Cavallo-Medved D, Sloane BF, Band V, Band H (2008) Lysosomal cathepsin B participates in the podosome-mediated extracellular matrix degradation and invasion via secreted lysosomes in v-Src fibroblasts. Cancer Res 68(22):9147–9156
Chang SH, Kanasaki K, Gocheva V, Blum G, Harper J, Moses MA, Shih SC, Nagy JA, Joyce J, Bogyo M, Kalluri R, Dvorak HF (2009) VEGF-A induces angiogenesis by perturbing the cathepsin-cysteine protease inhibitor balance in venules, causing basement membrane degradation and mother vessel formation. Cancer Res 69(10):4537–4544. doi:10.1158/0008-5472.CAN-08-4539
Burden RE, Gormley JA, Jaquin TJ, Small DM, Quinn DJ, Hegarty SM, Ward C, Walker B, Johnston JA, Olwill SA, Scott CJ (2009) Antibody-mediated inhibition of cathepsin S blocks colorectal tumor invasion and angiogenesis. Clin Cancer Res 15(19):6042–6051. doi:10.1158/1078-0432.CCR-09-1262
Perez-Castrillon JL, Pinacho F, De Luis D, Lopez-Menendez M, Duenas Laita A (2010) Odanacatib, a new drug for the treatment of osteoporosis: review of the results in postmenopausal women. J Osteoporos 2010. doi:10.4061/2010/401581
Doyle PS, Zhou YM, Engel JC, McKerrow JH (2007) A cysteine protease inhibitor cures Chagas’ disease in an immunodeficient-mouse model of infection. Antimicrob Agents Chemother 51(11):3932–3939. doi:10.1128/AAC.00436-07
Acknowledgments
Financial support for this research was provided by Nemours Research Programs, National Institutes of Health Grant 8P20GM103464, National Institutes of Health Grant P20RR016472 and Alex’s Lemonade Stand Foundation. We wish to thank Matthew R. England for initial assistance in proteomic studies, Dr. M. David Percival (Merck Frosst Centre for Therapeutic Research, Quebec, Canada) for generously providing the L-006235, L-625, and L-264 cathepsin inhibitory compounds and Dr. James McKerrow (University of California, San Francisco) for kindly providing cathepsin inhibitory agent K11777 and the rest of our colleagues for helpful discussions.
Conflict of interest
Authors have no financial or personal relationships with other people or organizations that could inappropriately influence this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cartledge, D.M., Colella, R., Glazewski, L. et al. Inhibitors of cathepsins B and L induce autophagy and cell death in neuroblastoma cells. Invest New Drugs 31, 20–29 (2013). https://doi.org/10.1007/s10637-012-9826-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-012-9826-6