Summary
Objective
The anti-vascular endothelial growth factor (VEGF) antibody bevacizumab has received considerable attention as a first-line treatment of advanced colorectal cancers. Difficulties associated with effectively monitoring the activity of this drug have prompted us to seek a pharmacodynamic marker suitable for defining the optimum biological dose and schedule of bevacizumab administration against colon cancer in early clinical trials.
Methods
We evaluated inhibitory effects of bevacizumab on VEGF signaling and tumor growth in vitro and in vivo, and assessed phosphorylation of VEGF receptor 2 (VEGFR2) and downstream signaling in endothelial cells as pharmacodynamic markers using phospho-flow cytometry. We also validated markers in patients with metastatic colorectal cancer (mCRC) treated with bevacizumab-based chemotherapy.
Results
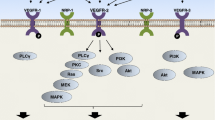
In in vitro studies, bevacizumab inhibited proliferation of human umbilical vein endothelial cells in association with reduced VEGF signaling. Notably, bevacizumab inhibited VEGF-induced phosphorylation of VEGFR-2, Akt, and extracellular signal-regulated kinase (ERK). In vivo, treatment with bevacizumab inhibited growth of xenografted tumors and attenuated VEGF-induced phosphorylation of Akt and ERK. The median percentages of VEGFR2 + pAkt + and VEGFR2 + pERK + cells, determined by phospho-flow cytometry, were approximately 3-fold higher in mCRC patients than in healthy controls. Bevacizumab treatment decreased VEGFR2 + pAkt + cells in 18 of 24 patients on day 3.
Conclusion
Bevacizumab combined with chemotherapy decreased the number of VEGFR2 + pAkt + cells, reflecting impaired VEGFR2 signaling. Together, these data suggest that changes in the proportion of circulating VEGFR2 + pAkt + cells may be a potential pharmacodynamic marker of the efficacy of antiangiogenic agents, and could prove valuable in determining drug dosage and administration schedule.






Similar content being viewed by others
References
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186. doi:10.1056/NEJM197111182852108
Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1(1):27–31
Jain RK (2001) Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7(9):987–989. doi:10.1038/nm0901-987
Kerbel RS (2000) Tumor angiogenesis: past, present and the near future. Carcinogenesis 21(3):505–515
Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26(12):2013–2019. doi:10.1200/JCO.2007.14.9930
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342. doi:10.1056/NEJMoa032691
Sessa C, Guibal A, Del Conte G, Ruegg C (2008) Biomarkers of angiogenesis for the development of antiangiogenic therapies in oncology: tools or decorations? Nat Clin Pract Oncol 5(7):378–391. doi:10.1038/ncponc1150
Poon RT, Fan ST, Wong J (2001) Clinical implications of circulating angiogenic factors in cancer patients. J Clin Oncol 19(4):1207–1225
Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS, Kabbinavar F, Holden SN, Novotny WF, Frantz GD, Hillan KJ, Koeppen H (2006) Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol 24(2):217–227. doi:10.1200/JCO.2005.01.5388
Bertolini F, Shaked Y, Mancuso P, Kerbel RS (2006) The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer 6(11):835–845. doi:10.1038/nrc1971
Mancuso P, Burlini A, Pruneri G, Goldhirsch A, Martinelli G, Bertolini F (2001) Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood 97(11):3658–3661
Shaked Y, Emmenegger U, Man S, Cervi D, Bertolini F, Ben-David Y, Kerbel RS (2005) Optimal biologic dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood 106(9):3058–3061. doi:10.1182/blood-2005-04-1422
Mancuso P, Colleoni M, Calleri A, Orlando L, Maisonneuve P, Pruneri G, Agliano A, Goldhirsch A, Shaked Y, Kerbel RS, Bertolini F (2006) Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood 108(2):452–459. doi:10.1182/blood-2005-11-4570
Hlatky L, Hahnfeldt P, Folkman J (2002) Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. J Natl Cancer Inst 94(12):883–893
Tofts PS (1997) Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging 7(1):91–101
Hale MB, Nolan GP (2006) Phospho-specific flow cytometry: intersection of immunology and biochemistry at the single-cell level. Curr Opin Mol Ther 8(3):215–224
Li Q, Yano S, Ogino H, Wang W, Uehara H, Nishioka Y, Sone S (2007) The therapeutic efficacy of anti vascular endothelial growth factor antibody, bevacizumab, and pemetrexed against orthotopically implanted human pleural mesothelioma cells in severe combined immunodeficient mice. Clin Cancer Res 13(19):5918–5925. doi:10.1158/1078-0432.CCR-07-0501
Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N (1997) Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res 57(20):4593–4599
Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF 3rd, Gaudreault J, Damico LA, Holmgren E, Kabbinavar F (2004) Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 22(11):2184–2191. doi:10.1200/JCO.2004.11.022
Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349(5):427–434. doi:10.1056/NEJMoa021491
Fox WD, Higgins B, Maiese KM, Drobnjak M, Cordon-Cardo C, Scher HI, Agus DB (2002) Antibody to vascular endothelial growth factor slows growth of an androgen-independent xenograft model of prostate cancer. Clin Cancer Res 8(10):3226–3231
Inoue S, Hartman A, Branch CD, Bucana CD, Bekele BN, Stephens LC, Chada S, Ramesh R (2007) mda-7 In combination with bevacizumab treatment produces a synergistic and complete inhibitory effect on lung tumor xenograft. Mol Ther 15(2):287–294. doi:10.1038/sj.mt.6300035
Monestiroli S, Mancuso P, Burlini A, Pruneri G, Dell'Agnola C, Gobbi A, Martinelli G, Bertolini F (2001) Kinetics and viability of circulating endothelial cells as surrogate angiogenesis marker in an animal model of human lymphoma. Cancer Res 61(11):4341–4344
Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, Faghih M, Brendel E, Voliotis D, Haase CG, Schwartz B, Awada A, Voigtmann R, Scheulen ME, Seeber S (2005) Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol 23(5):965–972. doi:10.1200/JCO.2005.06.124
Ludwig JA, Weinstein JN (2005) Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer 5(11):845–856. doi:10.1038/nrc1739
Acknowledgments
This study was supported by a grant of the Korea Health technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A090660) and a faculty research grant of Department of Internal Medicine, Yonsei University College of Medicine for 2009.
Disclosure Statement
The authors indicate no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shin, S.J., Hwang, J.W., Ahn, J.B. et al. Circulating vascular endothelial growth factor receptor 2/pAkt-positive cells as a functional pharmacodynamic marker in metastatic colorectal cancers treated with antiangiogenic agent. Invest New Drugs 31, 1–13 (2013). https://doi.org/10.1007/s10637-012-9817-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-012-9817-7