Summary
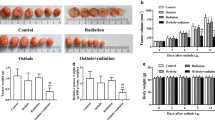
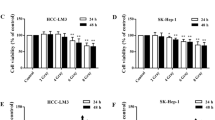
Previous studies have demonstrated that tyroserleutide (YSL) inhibits tumor growth in an animal model of hepatocellular carcinoma (HCC). However, its effects on HCC metastasis are still not fully understood. To examine YSL as a novel agent to prevent HCC metastasis, a metastatic human HCC orthotopic nude mouse model of MHCC97L was used. The antitumor and antimetastasis effects of YSL were also evaluated in combination with radiation. Hypoxia and epithelial-mesenchymal transition (EMT)-related molecules were studied. YSL inhibited MHCC97L cell invasion in vitro with or without irradiation. YSL did not significantly inhibit tumor growth but decreased pulmonary metastasis and prolonged life-span for more than 40 days, which correlated with down-regulation of matrix metalloproteinase-2. Radiotherapy inhibited early-stage tumor growth and promoted tumor hypoxia. The re-implanted tumor volume in the radiotherapy group was not significantly different from the control, in which the incidence of lung metastasis increased after radiotherapy (6/6 versus 3/6, P = 0.046); however, YSL inhibited the growth of re-implanted tumor after radiotherapy. Furthermore, YSL at 160 or 320 μg/kg/day almost completely inhibited lung metastasis induced by irradiation (1/6 versus 6/6, P = 0.002 for both dosages). YSL down-regulated hypoxia-inducible factor 1α (HIF-1α) and transmembrane protease serine 4 (TMPRSS4), and inhibited EMT was associated with the antimetastasis capability of YSL. Our data suggest that YSL inhibits the enhanced invasiveness and metastatic potential of HCC induced by irradiation through down-regulation of HIF-1α and TMPRSS4 and inhibition of EMT. YSL may have potential as a new antimetastasis agent for radiotherapy.






Similar content being viewed by others
Abbreviations
- EMT:
-
epithelial-mesenchymal transition
- HCC:
-
hepatocellular carcinoma
- HIF-1α:
-
hypoxia-inducible factor 1α
- MMP-2:
-
matrix metalloproteinase-2
- TMPRSS4:
-
transmembrane protease serine 4
- YSL:
-
tyroserleutide
References
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55(2):74–108. doi:55/2/74[pii]
Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362(9399):1907–1917. doi:10.1016/S0140-6736(03)14964-1
Steeg PS (2006) Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 12(8):895–904. doi:10.1038/nm1469
Tanaka K, Shimada H, Matsuo K, Takeda K, Nagano Y, Togo S (2008) Clinical features of hepatocellular carcinoma developing extrahepatic recurrences after curative resection. World J Surg 32(8):1738–1747. doi:10.1007/s00268-008-9613-x
Rashidi B, An Z, Sun FX, Li X, Tang ZY, Moossa AR, Hoffman RM (2001) Efficacy of intra-hepatectomy 5-FU on recurrence and metastasis of human hepatocellular carcinoma in nude mice. Int J Cancer 91(2):231–235. doi:10.1002/1097-0215(20010115)91
Rofstad EK, Mathiesen B, Galappathi K (2004) Increased metastatic dissemination in human melanoma xenografts after subcurative radiation treatment: radiation-induced increase in fraction of hypoxic cells and hypoxia-induced up-regulation of urokinase-type plasminogen activator receptor. Cancer Res 64(1):13–18
Yamauchi K, Yang M, Hayashi K, Jiang P, Yamamoto N, Tsuchiya H, Tomita K, Moossa AR, Bouvet M, Hoffman RM (2008) Induction of cancer metastasis by cyclophosphamide pretreatment of host mice: an opposite effect of chemotherapy. Cancer Res 68(2):516–520. doi:10.1158/0008-5472.CAN-07-3063
Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS (2009) Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15(3):232–239. doi:10.1016/j.ccr.2009.01.021
Zeng ZC, Fan J, Tang ZY, Zhou J, Wang JH, Wang BL, Guo W (2008) Prognostic factors for patients with hepatocellular carcinoma with macroscopic portal vein or inferior vena cava tumor thrombi receiving external-beam radiation therapy. Cancer Sci 99(12):2510–2517. doi:10.1111/j.1349-7006.2008.00981.x
Strunk HM (2009) Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma. Hepatology 50(2):652–653. doi:10.1002/hep.23027, author reply 3
Park W, Lim DH, Paik SW, Koh KC, Choi MS, Park CK, Yoo BC, Lee JE, Kang MK, Park YJ, Nam HR, Ahn YC, Huh SJ (2005) Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 61(4):1143–1150. doi:10.1016/j.ijrobp.2004.08.028
Cheng JC, Chou CH, Kuo ML, Hsieh CY (2006) Radiation-enhanced hepatocellular carcinoma cell invasion with MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction pathway. Oncogene 25(53):7009–7018. doi:10.1038/sj.onc.1209706
Yao Z, Lu R, Jia J, Zhao P, Yang J, Zheng M, Lu J, Jin M, Yang H, Gao W (2006) The effect of tripeptide tyroserleutide (YSL) on animal models of hepatocarcinoma. Peptides 27(6):1167–1172. doi:10.1016/j.peptides.2005.02.026
Yao Z, Che XC, Lu R, Zheng MN, Zhu ZF, Li JP, Jian X, Shi LX, Liu JY, Gao WY (2007) Inhibition by tyroserleutide (YSL) on the invasion and adhesion of the mouse melanoma cell. Mol Med 13(1–2):14–21. doi:10.2119/2006-00061
Tian J, Tang ZY, Ye SL, Liu YK, Lin ZY, Chen J, Xue Q (1999) New human hepatocellular carcinoma (HCC) cell line with highly metastatic potential (MHCC97) and its expressions of the factors associated with metastasis. Br J Cancer 81(5):814–821. doi:10.1038/sj.bjc.6690769
Tsukamoto H, Shibata K, Kajiyama H, Terauchi M, Nawa A, Kikkawa F (2007) Irradiation-induced epithelial-mesenchymal transition (EMT) related to invasive potential in endometrial carcinoma cells. Gynecol Oncol 107(3):500–504. doi:10.1016/j.ygyno.2007.08.058
Andarawewa KL, Erickson AC, Chou WS, Costes SV, Gascard P, Mott JD, Bissell MJ, Barcellos-Hoff MH (2007) Ionizing radiation predisposes nonmalignant human mammary epithelial cells to undergo transforming growth factor beta induced epithelial to mesenchymal transition. Cancer Res 67(18):8662–8670. doi:10.1158/0008-5472.CAN-07-1294
Ke AW, Shi GM, Zhou J, Wu FZ, Ding ZB, Hu MY, Xu Y, Song ZJ, Wang ZJ, Wu JC, Bai DS, Li JC, Liu KD, Fan J (2009) Role of overexpression of CD151 and/or c-Met in predicting prognosis of hepatocellular carcinoma. Hepatology 49(2):49–503. doi:10.1002/hep.22639
Wang L, Tang ZY, Qin LX, Wu XF, Sun HC, Xue Q, Ye SL (2000) High-dose and long-term therapy with interferon-alfa inhibits tumor growth and recurrence in nude mice bearing human hepatocellular carcinoma xenografts with high metastatic potential. Hepatology 32(1):43–48. doi:10.1053/jhep.2000.8525
Liu L, Ren ZG, Shen Y, Zhu XD, Zhang W, Xiong W, Qin Y, Tang ZY (2010) Influence of hepatic artery occlusion on tumor growth and metastatic potential in a human orthotopic hepatoma nude mouse model: Relevance of epithelial-mesenchymal transition. Cancer Sci 101(1):120–128. doi:10.1111/j.1349-7006.2009.01363.x
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408. doi:10.1006/meth.2001.1262
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC (2008) High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol 26(16):2707–2716. doi:10.1200/JCO.2007.15.6521
Hejna M, Raderer M, Zielinski CC (1999) Inhibition of metastases by anticoagulants. J Natl Cancer Inst 91(1):22–36
Gupta GP, Massague J (2006) Cancer metastasis: building a framework. Cell 127(4):679–695. doi:10.1016/j.cell.2006.11.001
Qin LX, Tang ZY (2002) The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol 8(3):385–392
Coussens LM, Fingleton B, Matrisian LM (2002) Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295(5564):2387–2392. doi:10.1126/science.1067100
Overall CM, Kleifeld O (2006) Tumour microenvironment—opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer 6(3):227–239. doi:10.1038/nrc1821
Seong J, Park HC, Han KH, Chon CY (2003) Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients. Int J Radiat Oncol Biol Phys 55(2):329–336. doi:S0360301602039299[pii]
Li B, Yu J, Wang L, Li C, Zhou T, Zhai L, Xing L (2003) Study of local three-dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for patients with stage III hepatocellular carcinoma. Am J Clin Oncol 26(4):e92–99. doi:10.1097/01.COC.0000077936.97997.AB
Murphy ED, Kaplan HS (1948) Enhancement of metastasis of a mouse mammary carcinoma following roentgen irradiation. Am J Pathol 24(3):673
von Essen CF (1991) Radiation enhancement of metastasis: a review. Clin Exp Metastasis 9(2):77–104
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA (2009) Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 119(6):1438–1449. doi:10.1172/JCI38019
Osada T, Sakamoto M, Ino Y, Iwamatsu A, Matsuno Y, Muto T, Hirohashi S (1996) E-cadherin is involved in the intrahepatic metastasis of hepatocellular carcinoma. Hepatology 24(6):1460–1467. doi:10.1053/jhep.1996.v24.pm0008938181
Wallrapp C, Hahnel S, Muller-Pillasch F, Burghardt B, Iwamura T, Ruthenburger M, Lerch MM, Adler G, Gress TM (2000) A novel transmembrane serine protease (TMPRSS3) overexpressed in pancreatic cancer. Cancer Res 60(10):2602–2606
Kebebew E, Peng M, Reiff E, Duh QY, Clark OH, McMillan A (2005) ECM1 and TMPRSS4 are diagnostic markers of malignant thyroid neoplasms and improve the accuracy of fine needle aspiration biopsy. Ann Surg 242(3):353–361. doi:00000658-200509000-00006[pii], discussion 61-63
Choi SY, Shin HC, Kim SY, Park YW (2008) Role of TMPRSS4 during cancer progression. Drug News Perspect 21(8):417–423. doi:10.1358/dnp.2008.21.8.1272135
Jung H, Lee KP, Park SJ et al (2008) TMPRSS4 promotes invasion, migration and metastasis of human tumor cells by facilitating an epithelial-mesenchymal transition. Oncogene 27(18):2635–2647. doi:10.1038/sj.onc.1210914
Moulder JE, Rockwell S (1984) Hypoxic fractions of solid tumors: experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys 10(5):695–712. doi:0360-3016(84)90301-8[pii]
Moeller BJ, Cao Y, Li CY, Dewhirst MW (2004) Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell 5(5):429–441. doi:S1535610804001151[pii]
Dewhirst MW, Cao Y, Moeller B (2008) Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer 8(6):425–437. doi:10.1038/nrc2397
Yan W, Fu Y, Tian D, Liao J, Liu M, Wang B, Xia L, Zhu Q, Luo M (2009) PI3 kinase/Akt signaling mediates epithelial-mesenchymal transition in hypoxic hepatocellular carcinoma cells. Biochem Biophys Res Commun 382(3):631–636. doi:10.1016/j.bbrc.2009.03.088
Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ (2008) Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol 10(3):295–305. doi:10.1038/ncb1691
Acknowledgements
The authors thank Dr. Zhen-Yu Zhang (Radiation Oncology, Zhongshan Hospital, Fudan University, Shanghai, China) for offering radiotherapy helps. This study was jointly supported by a China Postdoctoral Science Foundation–funded project (No. 20080440077 and 200902203) and a National Key Science and Technology Specific project (2008ZX10002-021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jin-Bin Jia and Wen-Quan Wang contributed equally to this work.
Jin-Bin Jia works for Shenzhen Kangzhe Pharmaceutical Co. Ltd. which owns the patent for tyroserleutide. The other authors have no financial disclosures or conflicts of interest to report.
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
Fig. S1
Reference markers of tumor hypoxia pimonidazole staining and HIF-1α expression in tumor tissue with or without irradiation treatment. Low (a) or high (b) pimonidazole staining, (50×). HIF-1α mRNA (c) and protein (d) level in control and radiotherapy group. *P < 0.05, compared with the control. β-actin was an internal control in Western blot analysis. (GIF 63 kb)
Fig. S2
Effects of YSL on weight loss in nude mice after radiotherapy. (GIF 32 kb)
Fig. S3
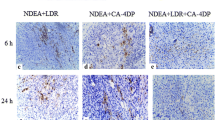
Effects of YSL on the expression of HIF-1α and TMPRSS4 analyzed by immunohistochemistry. a–e HIF-1α staining was mainly on the nuclei or cytoplasm of tumor cells. f–j TMPRSS4 staining was mainly on the cytomembrane of tumor cells. a and f showed high expression of the two molecules, and the others showed low expression of the two molecules. (GIF 613 kb)
Table S1
The differential expression of four tumor invasion and metastasis-related genes in tumor tissue different times after radiotherapy. (DOC 35 kb)
Rights and permissions
About this article
Cite this article
Jia, JB., Wang, WQ., Sun, HC. et al. A novel tripeptide, tyroserleutide, inhibits irradiation-induced invasiveness and metastasis of hepatocellular carcinoma in nude mice. Invest New Drugs 29, 861–872 (2011). https://doi.org/10.1007/s10637-010-9435-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9435-1