Summary
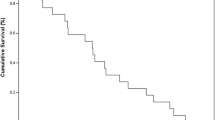
Purpose: To determine the safety and efficacy of weekly high-dose oral calcitriol and docetaxel, given to patients with non-resectable, incurable pancreatic cancer. Patients and Methods: Twenty-five patients were enrolled onto this phase II study. Patients were treated with oral calcitriol 0.5 μg/kg on day 1, followed by docetaxel 36 mg/m2 IV on day 2, administered weekly for three consecutive weeks, followed by 1 week without treatment. Patients followed a low-calcium diet and increased their hydration. The primary end-point of the trial was time-to-progression. Results: Three of 25 patients attained a partial response (12%, 95% CI 3 to 31) and seven (28%) achieved stable disease. Median time-to-progression was 15 weeks, and median overall survival was 24 weeks. Toxicities observed (hyperglycemia, fatigue) were mostly attributable to the docetaxel or its pre-treatment. Conclusions: This regimen of high-dose calcitriol with docetaxel may have activity in incurable pancreatic cancer, with a modest increase in TTP when compared to historical findings using single-agent docetaxel. However, results do not appear superior to those seen with gemcitabine, with or without erlotinib.
Similar content being viewed by others
References
Moore MJ, Goldstein D, Hamm J et al (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25:1960–1966 doi:10.1200/JCO.2006.07.9525
Lenzi R, Yalcin S, Evans DB et al (2002) Phase II study of docetaxel in patients with pancreatic cancer previously untreated with cytotoxic chemotherapy. Cancer Invest 20:464–472 doi:10.1081/CNV-120002146
Rougier P, Adenis A, Ducreux M et al (2000) A phase II study: docetaxel as first-line chemotherapy for advanced pancreatic adenocarcinoma. Eur J Cancer 36:1016–1025 doi:10.1016/S0959-8049(00)00072-1
Okada S, Sakata Y, Matsuno S et al (1999) Phase II study of docetaxel in patients with metastatic pancreatic cancer: a Japanese cooperative study. Br J Cancer 80:438–443 doi:10.1038/sj.bjc.6690375
Wang QM, Jones JB, Studzinski GP (1996) Cyclin-dependent kinase inhibitor p27 as a mediator of the G1-S phase block induced by 1,25-dihydroxyvitamin D3 in HL60 cells. Cancer Res 56:264–267
Liu M, Lee MH, Cohen M et al (1996) Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev 10:142–153 doi:10.1101/gad.10.2.142
Hershberger PA, Yu WD, Modzelewski RA et al (2001) Calcitriol (1,25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis. Clin Cancer Res 7:1043–1051
Pettersson F, Colston KW, Dalgleish AG (2000) Differential and antagonistic effects of 9-cis-retinoic acid and vitamin D analogues on pancreatic cancer cells in vitro. Br J Cancer 83:239–245 doi:10.1054/bjoc.2000.1281
Beer TM, Hough KM, Garzotto M et al (2001) Weekly high-dose calcitriol and docetaxel in advanced prostate cancer. Semin Oncol 28:49–55 doi:10.1016/S0093-7754(01)90155-1
Beer TM, Munar M, Henner WD (2001) A phase I trial of pulse calcitriol in patients with refractory malignancies: pulse dosing permits substantial dose escalation. Cancer 91:2431–2439 doi:10.1002/1097-0142(20010615)91:12<2431::AID-CNCR1278>3.0.CO;2-3
Beer TM, Ryan CW, Venner PM et al (2007) Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: a report from the ASCENT investigators. J Clin Oncol 25:669–674 doi:10.1200/JCO.2006.06.8197
Therasse P, Arbuck SG, Eisenhauer EA (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216 doi:10.1093/jnci/92.3.205
National Cancer Institute (1999) Cancer Therapy Evaluation Program: common toxicity criteria (version 2.0). National Cancer Institute, Bethesda
Shepard RC, Levy DE, Berlin JD et al (2004) Phase II study of gemcitabine in combination with docetaxel in patients with advanced pancreatic carcinoma (E1298). A trial of the Eastern Cooperative Oncology Group. Oncology 66:303–309 doi:10.1159/000078331
Johnson JA, Grande JP, Roche PC et al (1994) Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am J Physiol 267:E356–E360
Iseki K, Tatsuta M, Uehara H et al (1999) Inhibition of angiogenesis as a mechanism for inhibition by 1alpha-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 of colon carcinogenesis induced by azoxymethane in Wistar rats. Int J Cancer 81:730–733 doi:10.1002/(SICI)1097-0215(19990531)81:5<730::AID-IJC11>3.0.CO;2-Q
Wu K, Feskanich D, Fuchs CS et al (2007) A nested case-control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst 99:1120–1129 doi:10.1093/jnci/djm038
Lappe JM, Travers-Gustafson D, Davies KM et al (2007) Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr 85:1586–1591
Evans TRJ, Colston KW, Lofts FJ et al (2002) A phase II trial of the vitamin D analogue seocalcitol (EB1089) in patients with inoperable pancreatic cancer. Br J Cancer 86:680–685 doi:10.1038/sj.bjc.6600162
Novacea (2007) Novacea halts ASCENT-2 trial in advanced prostate cancer (press release). http://www.novacea.com/610.asp?id=136&nav=news
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blanke, C.D., Beer, T.M., Todd, K. et al. Phase II study of calcitriol-enhanced docetaxel in patients with previously untreated metastatic or locally advanced pancreatic cancer. Invest New Drugs 27, 374–378 (2009). https://doi.org/10.1007/s10637-008-9184-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-008-9184-6