Abstract
Background
Dystroglycanopathies are a heterogeneous group of membrane-related muscular dystrophies. The dystroglycanopathy phenotype includes a spectrum of severity ranging from severe congenital muscular dystrophy to adult-onset limb–girdle muscular dystrophy (LGMD). LGMDR9 is a dystroglycanopathy caused by mutations in the FKRP gene. Previous studies have characterized electroretinogram findings of dystroglycanopathy mouse models but have not been reported in humans.
Purpose
This study set out to characterize the electroretinogram in eight participants with LGMDR9.
Methods
Eight participants were recruited from an ongoing dystroglycanopathy natural history study at the University of Iowa (NCT00313677). Inclusion criteria for the current study were children and adults > 6 years old with confirmed LGMDR9. Age similar controls were identified from our electrophysiology service normative control database. Full-field electroretinograms were recorded using ISCEV standards. Six of the eight participants underwent light-adapted ON/OFF testing.
Results
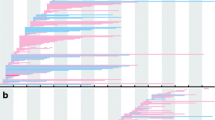
The electronegative electroretinogram was not seen in any participants with LGMDR9. An unusual sawtooth pattern in the 30 Hz flicker with faster rise than descent was noted in all 8 participants. Our cases showed a decreased b-wave amplitude in light-adapted ON responses (p = 0.011) and decreased d-wave amplitude in light-adapted OFF responses (p = 0.015). Decreased b-wave amplitude in light-adapted 3.0 testing (p = 0.015) and decreased flicker ERG amplitudes were also detected (p = 0.0018). Additionally, compared to controls, participants with LGMDR9 had decreased a-wave amplitudes on dark-adapted 10 testing (p = 0.026).
Conclusions
Abnormal ON/OFF bipolar cell responses and sawtooth 30 Hz flicker waveforms on full-field electroretinogram may be specific for LGMDR9. If confirmed in a larger population and if related to disease stage, these tests are potential biomarkers which could be useful as endpoints in clinical treatment trials.



Similar content being viewed by others
References
Muntoni F et al (2011) Muscular dystrophies due to glycosylation defects: diagnosis and therapeutic strategies. Curr Opin Neurol 24(5):437–442
Taniguchi-Ikeda M et al (2016) Mechanistic aspects of the formation of alpha-dystroglycan and therapeutic research for the treatment of alpha-dystroglycanopathy: A review. Mol Aspects Med 51:115–124
Kanagawa M et al (2016) Identification of a post-translational modification with ribitol-phosphate and its defect in muscular dystrophy. Cell Rep 14(9):2209–2223
Ervasti JM, Campbell KP (1993) A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol 122(4):809–823
Gee SH et al (1994) Dystroglycan-alpha, a dystrophin-associated glycoprotein, is a functional agrin receptor. Cell 77(5):675–686
Peng HB et al (1998) The relationship between perlecan and dystroglycan and its implication in the formation of the neuromuscular junction. Cell Adhes Commun 5(6):475–489
Sugita S et al (2001) A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol 154(2):435–445
Sato S et al (2008) Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat Neurosci 11(8):923–931
Yoshida-Moriguchi T, Campbell KP (2015) Matriglycan: a novel polysaccharide that links dystroglycan to the basement membrane. Glycobiology 25(7):702–713
Montanaro F et al (1995) Dystroglycan expression in the wild type and mdx mouse neural retina: synaptic colocalization with dystrophin, dystrophin-related protein but not laminin. J Neurosci Res 42(4):528–538
Jastrow H et al (2006) Identification of a beta-dystroglycan immunoreactive subcompartment in photoreceptor terminals. Invest Ophthalmol Vis Sci 47(1):17–24
Rubio-Fernandez M et al (2018) Impairment of photoreceptor ribbon synapses in a novel Pomt1 conditional knockout mouse model of dystroglycanopathy. Sci Rep 8(1):8543
Satz JS et al (2009) Visual impairment in the absence of dystroglycan. J Neurosci 29(42):13136–13146
Holzfeind PJ et al (2002) Skeletal, cardiac and tongue muscle pathology, defective retinal transmission, and neuronal migration defects in the Largemyd mouse defines a natural model for glycosylation-deficient muscle–eye–brain disorders. Hum Mol Genet 11(21):2673–2687
Cohn RD (2005) Dystroglycan: important player in skeletal muscle and beyond. Neuromuscul Disord 15(3):207–217
Brockington M et al (2001) Mutations in the fukutin-related protein gene (FKRP) identify limb girdle muscular dystrophy 2I as a milder allelic variant of congenital muscular dystrophy MDC1C. Hum Mol Genet 10(25):2851–2859
Beltran-Valero de Bernabé D et al (2004) Mutations in the FKRP gene can cause muscle-eye-brain disease and Walker-Warburg syndrome. J Med Genet 41(5):e61
Esapa CT et al (2002) Functional requirements for fukutin-related protein in the Golgi apparatus. Hum Mol Genet 11(26):3319–3331
Robson AG et al (2022) ISCEV Standard for full-field clinical electroretinography (2022 update). Doc Ophthalmol 144(3):165–177
Sustar M et al (2018) ISCEV extended protocol for the photopic On-Off ERG. Doc Ophthalmol 136(3):199–206
Robson AG et al (2018) ISCEV guide to visual electrodiagnostic procedures. Doc Ophthalmol 136(1):1–26
Jiang X et al (2021) Prevalence of electronegative electroretinograms in a healthy adult cohort. BMJ Open Ophthalmol 6(1):e000751
Thompson DA et al (2012) Retinal on-pathway deficit in congenital disorder of glycosylation due to phosphomannomutase deficiency. Arch Ophthalmol 130(6):712–719
Sieving PA (1993) Photopic ON- and OFF-pathway abnormalities in retinal dystrophies. Trans Am Ophthalmol Soc 91:701–773
Tremblay F et al (1994) Duchenne muscular dystrophy: negative scotopic bright-flash electroretinogram and normal dark adaptation. Can J Ophthalmol 29(6):280–283
Jensen H et al (1995) Duchenne muscular dystrophy negative electroretinograms and normal dark adaptation. Reappraisal of assignment of X linked incomplete congenital stationary night blindness. J Med Genet 32(5):348–351
Pillers DA et al (1995) mdxCv3 mouse is a model for electroretinography of Duchenne/Becker muscular dystrophy. Invest Ophthalmol Vis Sci 36(2):462–466
Pillers DA et al (1999) Duchenne/Becker muscular dystrophy: correlation of phenotype by electroretinography with sites of dystrophin mutations. Hum Genet 105(1–2):2–9
Ricotti V et al (2016) Ocular and neurodevelopmental features of Duchenne muscular dystrophy: a signature of dystrophin function in the central nervous system. Eur J Hum Genet 24(4):562–568
Orlandi C et al (2018) Transsynaptic binding of orphan receptor GPR179 to dystroglycan-pikachurin complex is essential for the synaptic organization of photoreceptors. Cell Rep 25(1):130-145.e5
Haro C et al (2018) Expression in retinal neurons of fukutin and FKRP, the protein products of two dystroglycanopathy-causative genes. Mol Vis 24:43–58
Uribe ML et al (2021) Retinal proteomics of a mouse model of dystroglycanopathies reveals molecular alterations in photoreceptors. J Proteome Res 20(6):3268–3277
Acknowledgements
Amanda Pfeifer provided technical assistance with creation of figures.
Funding
Ronald Keech Professorship (Drack), University of Iowa ERG fund (Drack, Pfeifer), Research to Prevent Blindness (All Authors). Natural history study (Mathews) supported by the NIH through the Iowa Wellstone Muscular Dystrophy Cooperative Research Center (U54 NS053672) and ICTS Clinical Research Unit by citing grant UL1TR002537.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interests
Not applicable.
Ethics approval
This prospective study was approved by the Institutional Review Board of the University of Iowa (IRB ID #201805774) and conformed to the requirements of the United States Health Insurance Portability and Privacy Act.
Informed consent
All subjects freely consented to participate in this study, and an informed consent was obtained from all participants included in the study.
Statement of human rights
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the University of Iowa Institutional Review Board (Approval Date: 6/18/18; IRB Approval Number: 201805774) and in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Statement on the welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hagedorn, J.L., Dunn, T.M., Bhattarai, S. et al. Electroretinogram abnormalities in FKRP-related limb–girdle muscular dystrophy (LGMDR9). Doc Ophthalmol 146, 7–16 (2023). https://doi.org/10.1007/s10633-022-09909-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-022-09909-4