Abstract
Purpose
Atropine eye drops are a common and effective treatment for slowing myopia progression, but the site and mode of action of atropine in controlling myopia are unclear. We investigated the early retinal sites of action of atropine by examining its effects on the human full-field electroretinogram (ffERG).
Method
Baseline ffERGs were recorded in both eyes of 24 healthy subjects (mean ± SD: 21.0 ± 2.3 years; spherical equivalent refraction, range: + 1.63 to − 0.75 D) using 6 standard ISCEV protocols, 30 min after bilateral pupil dilation with 1% Tropicamide. Atropine (1 drop, 0.1%) was then instilled into the non-dominant eye. 24 h later, ffERGs were again recorded in both eyes. Ratios (post-atropine: pre-atropine) of dark-adapted (DA) and light-adapted (LA) ffERGs were compared between atropine-treated and control eyes using multivariate repeated measures general linear models.
Results
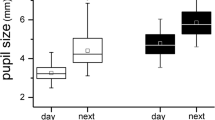
Atropine-treated eyes responded with 14% lower DA3.0 OP (oscillatory potential) amplitude (p = 0.003) and 4% delay in the DA10.0 a-wave peak time (p = 0.00099) compared with control eyes. Amplitudes and peak times were not different between atropine-treated and control eyes for DA0.01, LA3.0, and LA3.0 flicker ERGs. While atropine caused a small (1.26 mm2, p = 0.03) extra increase in pupil area in the treated eye, atropine-induced changes in ffERG responses bore no relationship with changes in pupil area (R2 = 2–5%, p > 0.05).
Conclusions
The observed changes in oscillatory potentials corroborate previous findings that atropine affects neural activity in the inner retina. However, observed changes to the a-wave suggest that atropine also affects activity in photoreceptors.





Similar content being viewed by others
References
Holden BA, Fricke TR, Wilson DA et al (2016) Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 123:1036–1042
Flitcroft DI (2012) The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res 31:622–660
Ohno-Matsui K, Lai TYY, Lai C-C, Cheung CMG (2016) Updates of pathologic myopia. Prog Retin Eye Res 52:156–187
Ma X, Zhou Z, Yi H et al (2014) Effect of providing free glasses on children’s educational outcomes in China: cluster randomized controlled trial. BMJ 349:g5740
Smith TST, Frick KD, Holden BA et al (2009) Potential lost productivity resulting from the global burden of uncorrected refractive error. Bull World Health Organ 87:431–437
Wu P-C, Chen C-T, Lin K-K et al (2018) Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology 125:1239–1250
He X, Sankaridurg P, Xiong S et al (2019) Shanghai time outside to reduce myopia trial: design and baseline data. Clin Experiment Ophthalmol 47:171–178
Yam JC, Jiang Y, Tang SM et al (2019) Low-concentration atropine for myopia progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology 126:113–124
Chamberlain P, Peixoto-de-Matos SC, Logan NS et al (2019) A 3-year randomized clinical trial of misight lenses for myopia control. Optom Vis Sci 96:556–567
Brennan NA (2012) Predicted reduction in high myopia for various degrees of myopia control. Cont Lens Anterior Eye 35:e14–e15
Bullimore MA, Brennan NA (2019) Myopia control: why each diopter matters. Optom Vis Sci 96:463–465
Chia A, Chua WH, Cheung YB et al (2012) Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (atropine for the treatment of myopia 2). Ophthalmology 119:347–354
Huang J, Wen D, Wang Q et al (2016) Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology 123:697–708
Chia A, Lu Q-SS, Tan DTH (2016) Five-Year clinical trial on atropine for the treatment of myopia 2 myopia control with atropine 0.01% eyedrops. Ophthalmology 123:391–399
Stone RA, Lin T, Laties AM (1991) Muscarinic antagonist effects on experimental chick myopia. Exp Eye Res 52:755–758
Schwahn HN, Kaymak H, Schaeffel F (2000) Effects of atropine on refractive development, dopamine release, and slow retinal potentials in the chick. Vis Neurosci 17:165–176
Carr BJ, Stell WK (2016) Nitric oxide (NO) mediates the inhibition of form-deprivation myopia by atropine in chicks. Sci Rep 6:9
Barathi VA, Chaurasia SS, Poidinger M et al (2014) Involvement of GABA transporters in atropine-treated myopic retina as revealed by iTRAQ quantitative proteomics. J Proteome Res 13:4647–4658
Fischer AJ, Miethke P, Morgan IG, Stell WK (1998) Cholinergic amacrine cells are not required for the progression and atropine-mediated suppression of form-deprivation myopia. Brain Res 794:48–60
Gallego P, Martínez-García C, Pérez-Merino P et al (2012) Scleral changes induced by atropine in chicks as an experimental model of myopia. Ophthalmic Physiol Opt 32:478–484
Lind GJ, Chew SJ, Marzani D, Wallman J (1998) Muscarinic acetylcholine receptor antagonists inhibit chick scleral chondrocytes. Invest Ophthalmol Vis Sci 39:2217–2231
Carr BJ, Mihara K, Ramachandran R et al (2018) Myopia-inhibiting concentrations of muscarinic receptor antagonists block activation of alpha2a-adrenoceptors in vitro. Invest Ophthalmol Vis Sci 59:2778–2791
Zhang Z, Zhou Y, Xie Z et al (2016) The effect of topical atropine on the choroidal thickness of healthy children. Sci Rep 6:34936
Chiang STH, Phillips JR (2018) Effect of atropine eye drops on choroidal thinning induced by hyperopic retinal defocus. J Ophthalmol 2018:1–6
Robson AG, Nilsson J, Li S et al (2018) ISCEV guide to visual electrodiagnostic procedures. Doc Ophthalmol 136:1–26
Khanal S, Turnbull PRK, Lee N, Phillips JR (2019) The effect of atropine on human global flash mfERG responses to retinal defocus. Invest Ophthalmol Vis Sci 60:218–225
McCulloch DL, Marmor MF, Brigell MG et al (2015) ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130:1–12
Hamilton R, Al Abdlseaed A, Healey J et al (2015) Multi-centre variability of ISCEV standard ERGs in two normal adults. Doc Ophthalmol 130:83–101
Anders LM, Heinrich SP, Lagrèze WA, Joachimsen L (2019) Little effect of 0. 01% atropine eye drops as used in myopia prevention on the pattern electroretinogram. Doc Ophthalmol 138(2):1–11
Wachtmeister L (1998) Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res 17:485–521
Heynen H, Van Norren D (1985) Origin of the electroretinogram in the intact macaque eye—II: Current source-density analysis. Vision Res 25:709–715
Zhong X, Ge J, Smith EL, Stell WK (2004) Image defocus modulates activity of bipolar and amacrine cells in macaque retina. Invest Ophthalmol Vis Sci 45:2065–2074
Fischer AJ, McGuire JJ, Schaeffel F, Stell WK (1999) Light- and focus-dependent expression of the transcription factor ZENK in the chick retina. Nat Neurosci 2:706–712
Guo SS, Sivak JG, Callender MG, Diehl-Jones B (1995) Retinal dopamine and Lens-induced refractive errors in chicks. Curr Eye Res 14:385–389
Mani A, Schwartz GW, Toda H et al (2017) Circuit mechanisms of a retinal ganglion cell with stimulus-dependent response latency and activation beyond its dendrites. Curr Biol 27:471–482
Ho WC, Wong OY, Chan YC et al (2012) Sign-dependent changes in retinal electrical activity with positive and negative defocus in the human eye. Vision Res 52:47–53
Shimada Y, Bearse MA, Sutter EE (2005) Multifocal electroretinograms combined with periodic flashes: Direct responses and induced components. Graefes Arch Clin Exp Ophthalmol 243:132–141
Feldkaemper MP, Schaeffel F (2013) An updated view on the role of dopamine in myopia. Exp Eye Res 114:106–119
Stone RA, Lin T, Laties AM, Iuvone PM (1989) Retinal dopamine and form-deprivation myopia. Neurobiology 86:704–706
Miller RF, Dacheux RF, Frumkes TE (1977) Amacrine cells in necturus retina: evidence for independent gamma-aminobutyric acid-and glycine-releasing neurons. Science 198:748–750
Wachtmeister L, Dowling JE (1978) The oscillatory potentials of the mudpuppy retina. Invest Ophthalmol Vis Sci 17:1176–1188
Wachtmeister L (1980) Further studies of the chemical sensitivity of the oscillatory potentials of the electroretinogram (ERG) I. GABA-and glycine antagonists Acta Ophthalmol 58:712–725
Stone RA, Laties AM, Raviola E, Wiesel TN (1988) Increase in retinal vasoactive intestinal polypeptide after eyelid fusion in primates. Proc Natl Acad Sci U S A 85:257–260
Pickett Seltner RL, Stell WK (1995) The effect of vasoactive intestinal peptide on development of form deprivation myopia in the chick: a pharmacological and immunocytochemical study. Vision Res 35:1265–1270
Zhou X, Pardue MT, Iuvone PM, Qu J (2017) Dopamine signaling and myopia development: what are the key challenges. Prog Retin Eye Res 61:60–71
Granit R (1933) The components of the retinal action potential in mammals and their relation to the discharge in the optic nerve. J Physiol 77:207–239
Penn RD, Hagins WA (1969) Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature 223:201–204
Robson JG, Frishman LJ (2014) The rod-driven a-wave of the dark-adapted mammalian electroretinogram. Prog Retin Eye Res 39:1–22
Chia A, Li W, Tan D, Luu CD (2013) Full-field electroretinogram findings in children in the atropine treatment for myopia (ATOM2) study. Doc Ophthalmol 126:177–186
Luu CD, Lau AMI, Koh AHC, Tan D (2005) Multifocal electroretinogram in children on atropine treatment for myopia. Br J Ophthalmol 89:151–153
Crewther DP (2000) The role of photoreceptors in the control of refractive state. Prog Retin Eye Res 19:421–457
Rucker FJ (2013) The role of luminance and chromatic cues in emmetropisation. Ophthalmic Physiol Opt 33:196–214
Liang H, Crewther DP, Crewther SG, Barila AM (1995) A role for photoreceptor outer segments in the induction of deprivation myopia. Vision Res 35:1217–1225
Norton TT, Amedo AO, Siegwart JT (2006) Darkness causes myopia in visually experienced tree shrews. Investigative Opthalmol Visual Sci 47:4700
Wang Y, Xu Y, Liu X et al (2018) Amblyopia, strabismus and refractive errors in congenital ptosis: a systematic review and meta-analysis. Sci Rep 8:8320
He W, Sun T, Yang J et al (2017) Analysis of factors associated with the ocular features of congenital cataract children in the shanghai pediatric cataract study. J Ophthalmol 2017:8647435
Morgan IG, Boelen MK (1996) A retinal dark-light switch: a review of the evidence. Vis Neurosci 13:399–409
Acknowledgements
The authors thank the New Zealand Association of Optometrists for a Higher Degree Research Write-up Scholarship to SK
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Safal Khanal’s contributions included Conceptualization, Methodology, Formal analysis, Visualisation, Writing – Original Draft. Sachi Rathod’s contributions included Investigation, Data Curation, Project Administration. John Phillips’ contributions included Conceptualization, Supervision, Resources, Writing – Review and Editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest or commercial relationship in relation to this work.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that all participants provided informed consent for publication of the study findings. The study does not report identifying information for any participants.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Human Participants Ethics Committee of the University of Auckland (ref: 017982).
Statement on the welfare of animals
No animals were used in this research.
Disclosure
This study was presented in part at the 17th International Myopia Conference, Tokyo, Japan, September 2019. Author SK was the recipient of a University of Auckland PhD scholarship funded by CooperVision Inc. Author SNR was supported by a summer research scholarship awarded by the Faculty of Medical and Health Science, University of Auckland.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khanal, S., Rathod, S.N. & Phillips, J.R. The acute effect of atropine eye drops on the human full-field electroretinogram. Doc Ophthalmol 142, 315–328 (2021). https://doi.org/10.1007/s10633-020-09806-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-020-09806-8