Abstract
Purpose
As part of a long-term, prospective study of prenatal and clinical risk factors for optic nerve hypoplasia (ONH) at Children’s Hospital Los Angeles, pattern ERGs (PERGs) were evaluated for prognostic value using an automated objective and robust analytical method.
Methods
Participants were 33 children with ophthalmoscopically diagnosed ONH [disc diameter-to-disc macula ratio (DD/DM) less than 0.35 in one or both eyes on fundus photographs]. Using cycloplegia and chloral hydrate sedation in one session before 26 months of age, we recorded PERGs to checkerboard reversal using five check sizes. Participants were followed with clinical and psychometric testing until 5 years of age. PERGs were analysed using automated robust statistics based on magnitude-squared coherence and bootstrapping optimized to objectively quantify PERG recovery in the challenging recordings encountered in young patients. PERG measures in the fixating or better-seeing eyes were compared with visual outcome data.
Results
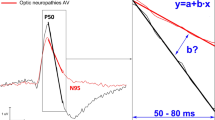
PERG recording was complete to at least three check sizes in all eyes and to all five sizes in 79%. Probability of recording a PERG that is significantly different from noise varied with check size from 73% for the largest checks to 30% for the smallest checks (p = 0.002); smaller waveforms were associated with earlier implicit times. The presence of significant PERGs in infancy is associated with better visual outcomes; the strongest association with visual outcome was for the threshold check size with a significant N95 component (ρ = 0.398, p = 0.02).
Conclusions
Automated statistically robust signal-processing techniques reliably and objectively detect PERGs in young children with ONH and show that congenital deficits of retinal ganglion cells are associated with diminished or non-detectable PERGs. The later negativity, N95, was the best indicator of visual prognosis and was most useful to identify those with good visual outcomes (≤0.4 LogMAR). Although PERGs reflect function of the inner layers of the central retina, they lack the specificity required to determine prognosis reliably in individual cases.

Similar content being viewed by others
Notes
This cohort does not include any cases published by laboratory in previous studies.
References
Garcia-Filion P, Borchert M (2013) Optic nerve hypoplasia syndrome: a review of the epidemiology and clinical associations. Curr Treat Options Neurol 15(1):78–89
Goh Y, Andrew D, Mcghee C, Dai S (2014) Clinical and demographic associations with optic nerve hypoplasia in New Zealand. Br J Ophthalmol 98(10):1364–1367
Mohney B, Young R, Diehl N (2013) Incidence and associated endocrine and neurologic abnormalities of optic nerve hypoplasia. Arch Ophthalmol 131(7):898–902
Ryabets-Lienhard A, Stewart C, Borchert M, Geffner ME (2016) The optic nerve hypoplasia spectrum: review of the literature and clinical guidelines. Adv Pediatr 63(1):127
Skarf B, Hoyt CS (1984) Optic-nerve hypoplasia in children—association with anomalies of the endocrine and CNS. Arch Ophthalmol 102:62–67
Garcia-Filion P, Epport K, Nelson M et al (2008) Neuroradiographic, endocrinologic, and ophthalmic correlates of adverse developmental outcomes in children with optic nerve hypoplasia: a prospective study. Pediatrics 121:E653–E659
Zeki SM, Hollman AS, Dutton GN (1992) Neuroradiological features of patients with optic-nerve hypoplasia. J Pediatr Ophthalmol 29:107–112
Hoyt CS, Billson FA (1986) Optic-nerve hypoplasia—changing perspectives. Clin Exp Ophthalmol 14:325–331
Ahmad T, Garcia-Filion P, Borchert M, Kaufman F, Burkett L, Geffner M (2006) Endocrinological and auxological abnormalities in young children with optic nerve hypoplasia: a prospective study. J Pediatr 148:78–84
Cemeroglu AP, Coulas T, Kleis L (2015) Spectrum of clinical presentations and endocrinological findings of patients with septo-optic dysplasia: a retrospective study. J Pediatr Endocrinol Metab 28(9–10):1057–1063
Garcia-Filion P (2011) An analysis of the prenatal history associated with optic nerve hypoplasia. Unpublished PhD disertation
Mcculloch DL, Borchert MS (1995) The pattern electroretinogram (erg) in infants with optic-nerve hypoplasia (onh). Invest Ophthalmol Vis Sci 36(4):S454
McCulloch DL, Garcia-Filion P, Fink C, Chaplin CA, Borchert MS (2010) Clinical electrophysiology and visual outcome in optic nerve hypoplasia. Br J Ophthalmol 94:1017–1023
Stewart C, Garcia-Filion P, Fink C, Ryabets-Lienhard A, Geffner ME, Borchert M (2016) Efficacy of growth hormone replacement on anthropometric outcomes, obesity, and lipids in children with optic nerve hypoplasia and growth hormone deficiency. Int J Pediatr Endocrinol 2016:5
Holder GE (2001) Pattern electroretinography (PERG) and an integrated approach to visual pathway diagnosis. Prog Retin Eye Res 20:531–561
Luo XD, Frishman LJ (2011) Retinal pathway origins of the pattern electroretinogram (PERG). Investig Ophthalmol Vis Sci 52:8571–8584
Bach M (2001) Electrophysiological approaches for early detection of glaucoma. Eur J Ophthalmol 11:S41–S49
Bach M, Gerling J, Geiger K (1992) Optic atrophy reduces the pattern-electroretinogram for both fine and coarse stimulus patterns. Clin Vis Sci 7:327–333
Harrison JM, Oconnor PS, Young RSL, Kincaid M, Bentley R (1987) The pattern erg in man following surgical resection of the optic-nerve. Investig Ophthalmol Vis Sci 28:492–499
Poloschek CM, Bach M (2012) Electrophysiological examination methods in glaucoma diagnostics. Ophthalmologe 109:358–363
Preiser D, Lagreze WA, Bach M, Poloschek CM (2013) Photopic negative response versus pattern electroretinogram in early glaucoma. Investig Ophthalmol Vis Sci 54:1182–1191
Rangaswamy NV, Frishman LJ, Dorotheo EU, Schiffman JS, Bahrani HM, Tang RA (2004) Photopic ERGs in patients with optic neuropathies: comparison with primate ERGs after pharmacologic blockade of inner retina. Investig Ophthalmol Vis Sci 45:3827–3837
Porciatti V (2014) Electrophysiological assessment of retinal ganglion cell function. Exp Eye Res 141:164–170
Holder GE, Votruba M, Carter AC, Bhattacharya SS, Fitzke FW, Moore AT (1998) Electrophysiological findings in dominant optic atrophy (DOA) linking to the OPA1 locus on chromosome 3q 28-qter. Doc Ophthalmol 95:217–228
McCulloch DL, Garcia-Fillion P, van Boemel GB, Borchert MS (2007) Retinal function in infants with optic nerve hypoplasia: electroretinograms to large patterns and photopic flash. Eye 21:712–720
McCulloch DL, Garcia-Filion P, van Boemel GB, Borchert MS (2010) Retinal function in infants with optic nerve hypoplasia: electroretinograms to large patterns and photopic flash. Eye 24:400
Fisher A, McCulloch D, Borchert M et al (2015) Comparison of human expert and computer-automated systems using magnitude-squared coherence (MSC) and bootstrap distribution statistics for the interpretation of pattern electroretinograms (PERGs) in infants with optic nerve hypoplasia (ONH). Doc Ophthalmol 131(1):25–34
Bach M, Brigell MG, Hawlina M et al (2013) ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc Ophthalmol 126:1–7
McCulloch D, Marmor M, Brigell M et al (2015) ISCEV standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130(1):1–12
Borchert M, McCulloch D, Rother C, Stout AU (1995) Clinical-assessment, optic disk measurements, and visual-evoked potential in optic-nerve hypoplasia. Am J Ophthalmol 120:605–612
Dawson WW, Trick GL, Litzkow CA (1979) Improved electrode for electroretinography. Investig Ophthalmol Vis Sci 18:988–991
McCulloch DL, Van Boemel GB, Borchert MS (1997) Comparisons of contact lens, foil, fiber and skin electrodes for patterns electroretinograms. Doc Ophthalmol 94:327–340
Barnett V, Lewis T (1994) Outliers in statistical data. Wiley, Chichester
Efron B, Tibshirani R (1993) An introduction to the bootstrap. Chapman & Hall/CRC, Boca Raton
Hoyt CS, Good WV (1992) Do we really understand the difference between optic-nerve hypoplasia and atrophy. Eye 6:201–204
Kelly J, Phillips J, Weiss A (2016) VEP analysis methods in children with optic nerve hypoplasia: relationship to visual acuity and optic disc diameter. Doc Ophthalmol 133(3):159–169
Thompson DA, Drasdo N (1994) The origins of luminance and pattern responses of the pattern electroretinogram. Int J Psychophysiol 16:219–227
Viswanathan S, Frishman LJ, Robson JG (2000) The uniform field and pattern ERG in macaques with experimental glaucoma: removal of spiking activity. Investig Ophthalmol Vis Sci 41:2797–2810
Miura G, Wang MH, Ivers KM, Frishman LJ (2009) Retinal pathway origins of the pattern ERG of the mouse. Exp Eye Res 89(1):49–62
Patel A, Purohit R, Lee H et al (2016) Optic nerve head development in healthy infants and children using handheld spectral-domain optical coherence tomography. Ophthalmology 123(10):2147–2157
Kriss A, Russelleggitt I (1992) Electrophysiological assessment of visual pathway function in infants. Eye 6:145–153
Janaky M, Deak A, Pelle Z, Benedek G (1994) Electrophysiologic alterations in patients with optic-nerve hypoplasia. Doc Ophthalmol 86:247–257
Sprague JB, Wilson WB (1981) Electrophysiologic findings in bilateral optic-nerve hypoplasia. Arch Ophthalmol 99:1028–1029
Brecelj J, Stirn-Kranjc B (2004) Visual electrophysiological screening in diagnosing infants with congenital nystagmus. Clin Neurophysiol 115:461–470
Odom JV, Bach M, Brigell M et al (2010) ISCEV standard for clinical visual evoked potentials (2009 update). Doc Ophthalmol 120:111–119
Drasdo N (1986) The effect of perimetric stimulation on evoked potential distribution—a theoretical model. Ophthalmic Physiol Opt 6(3):269–274
Geer I, Spafford M (1994) Effect of experimental scotoma size and shape on the binocular and monocular pattern visual evoked potential. Doc Ophthalmol 86(3):295–310
Hood DC, Ghadiali Q, Zhang JC, Graham NV, Wolfson SS, Zhang X (2006) Contrast-response functions for multifocal visual evoked potentials: a test of a model relating V1 activity to multifocal visual evoked potentials activity. J Vis 6(5):580
Slotnick SD, Klein SA, Carney T, Sutter EE (2001) Electrophysiological estimate of human cortical magnification. Clin Neurophysiol 112(7):1349–1356
Acknowledgements
We thank the participants the clinical registry and their families.
Funding
This work is supported by One Small Voice Foundation and the Children’s Hospital Los Angeles grant UL1TR000130, from the National Center for Advancing Translational Sciences (NCATS) at the NIH. The sponsors had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony, or patent-licensing arrangements), or conflicting non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments.
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments. The article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from parents or legal guardians for all individual participants included in the study.
Rights and permissions
About this article
Cite this article
McCulloch, D., Garcia-Filion, P., Fink, C. et al. Predictive value of N95 waveforms of pattern electroretinograms (PERGs) in children with optic nerve hypoplasia (ONH). Doc Ophthalmol 135, 97–106 (2017). https://doi.org/10.1007/s10633-017-9603-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-017-9603-0