Abstract
Purpose
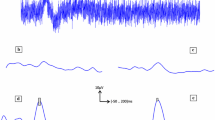
Pattern electroretinograms (PERGs) have inherently low signal-to-noise ratios and can be difficult to detect when degraded by pathology or noise. We compare an objective system for automated PERG analysis with expert human interpretation in children with optic nerve hypoplasia (ONH) with PERGs ranging from clear to undetectable.
Methods
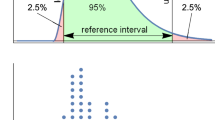
PERGs were recorded uniocularly with chloral hydrate sedation in children with ONH (aged 3.5–35 months). Stimuli were reversing checks of four sizes focused using an optical system incorporating the cycloplegic refraction. Forty PERG records were analysed; 20 selected at random and 20 from eyes with good vision (fellow eyes or eyes with mild ONH) from over 300 records. Two experts identified P50 and N95 of the PERGs after manually deleting trials with movement artefact, slow-wave EEG (4–8 Hz) or other noise from raw data for 150 check reversals. The automated system first identified present/not-present responses using a magnitude-squared coherence criterion and then, for responses confirmed as present, estimated the P50 and N95 cardinal positions as the turning points in local third-order polynomials fitted in the −3 dB bandwidth [0.25 … 45] Hz. Confidence limits were estimated from bootstrap re-sampling with replacement. The automated system uses an interactive Internet-available webpage tool (see http://clinengnhs.liv.ac.uk/esp_perg_1.htm).
Results
The automated system detected 28 PERG signals above the noise level (p ≤ 0.05 for H0). Good subjective quality ratings were indicative of significant PERGs; however, poor subjective quality did not necessarily predict non-significant signals. P50 and N95 implicit times showed good agreement between the two experts and between experts and the automated system. For the N95 amplitude measured to P50, the experts differed by an average of 13 % consistent with differing interpretations of peaks within noise, while the automated amplitude measure was highly correlated with the expert measures but was proportionally larger. Trial-by-trial review of these data required approximately 6.5 h for each human expert, while automated data processing required <4 min, excluding overheads relating to data transfer.
Conclusions
An automated computer system for PERG analysis, using a panel of signal processing and statistical techniques, provides objective present/not-present detection and cursor positioning with explicit confidence intervals. The system achieves, within an efficient and robust statistical framework, estimates of P50 and N95 amplitudes and implicit times similar to those of clinical experts.




Similar content being viewed by others
References
Holder GE (2001) Pattern electroretinography (PERG) and an integrated approach to visual pathway diagnosis. Prog Retin Eye Res 20(4):531–561
Luo XD, Frishman LJ (2011) Retinal pathway origins of the pattern electroretinogram (PERG). Invest Ophthalmol Vis Sci 52(12):8571–8584
Holder GE et al (1998) Electrophysiological findings in dominant optic atrophy (DOA) linking to the OPA1 locus on chromosome 3q 28-qter. Doc Ophthalmol 95(3–4):217–228
Harrison JM et al (1987) The pattern erg in man following surgical resection of the optic-nerve. Invest Ophthalmol Vis Sci 28(3):492–499
Lambert SR, Hoyt CS, Narahara MH (1987) Optic-nerve hypoplasia. Surv Ophthalmol 32(1):1–9
Borchert M et al (1995) Clinical-assessment, optic disk measurements, and visual-evoked potential in optic-nerve hypoplasia. Am J Ophthalmol 120(5):605–612
Hoyt CS, Billson FA (1986) Optic-nerve hypoplasia—changing perspectives. Aust N Z J Ophthalmol 14(4):325–331
Skarf B, Hoyt CS (1984) Optic-nerve hypoplasia in children—association with anomalies of the endocrine and CNS. Arch Ophthalmol 102(1):62–67
Zeki SM, Dudgeon J, Dutton GN (1991) Reappraisal of the ratio of disk to macula disk diameter in optic-nerve hypoplasia. Br J Ophthalmol 75(9):538–541
Janaky M et al (1994) Electrophysiologic alterations in patients with optic-nerve hypoplasia. Doc Ophthalmol 86(3):247–257
Hoyt CS, Good WV (1992) Do we really understand the difference between optic-nerve hypoplasia and atrophy. Eye 6:201–204
Ouvrier R, Billson F (1986) Optic-nerve hypoplasia—a review. J Child Neurol 1(3):181–188
Kriss A, Russelleggitt I (1992) Electrophysiological assessment of visual pathway function in infants. Eye 6:145–153
McCulloch DL et al (2007) Retinal function in infants with optic nerve hypoplasia: electroretinograms to large patterns and photopic flash. Eye 21(6):712–720
McCulloch DL et al (2010) Clinical electrophysiology and visual outcome in optic nerve hypoplasia. Br J Ophthalmol 94(8):1017–1023
Garcia-Filion P et al (2008) Neuroradiographic, endocrinologic, and ophthalmic correlates of adverse developmental outcomes in children with optic nerve hypoplasia: a prospective study. Pediatrics 121(3):E653–E659
Chaplin C et al (2009) Light-adapted electroretinograms in optic nerve hypoplasia. Doc Ophthalmol 119(2):123–132
Dawson WW, Trick GL, Litzkow CA (1979) Improved electrode for electroretinography. Invest Ophthalmol Vis Sci 18(9):988–991
McCulloch DL, Van Boemel GB, Borchert MS (1997) Comparisons of contact lens, foil, fiber and skin electrodes for patterns electroretinograms. Doc Ophthalmol 94(4):327–340
Bach M et al (2013) ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc Ophthalmol 126(1):1–7
Shoelson B (shoelson@helix.nih.gov). [cited 2013 04 March 2013]. http://clinengnhs.liv.ac.uk/ACF_Grubbs.htm
Barnett V, Lewis T (1994) Outliers in statistical data. Wiley Series in probability and Mathematical Statistics. Wiley, Chichester
Carter GC, Knapp CH, Nuttall AH (1973) Estimation of magnitude-squared coherence function via overlapped fast Fourier-transform processing. IEEE Trans Audio Electroacoust 21(4):337–344
Dobie RA, Wilson MJ (1989) Analysis of auditory evoked-potentials by magnitude-squared coherence. Ear Hear 10(1):2–13
Oppenheim AVS, Schafer RW (1975) Digital signal processing. Prentice-Hall International, Hemel Hempstead
Koopmans LH (1974) The spectral analysis of time series. Academic Press, San Diego
Efron B, Tibshirani R (1993) An introduction to the bootstrap. Chapman & Hall/CRC, Boca Raton
Viswanathan S, Frishman LJ, Robson JG (2000) The uniform field and pattern ERG in macaques with experimental glaucoma: removal of spiking activity. Invest Ophthalmol Vis Sci 41(9):2797–2810
Bland JM, Altman DG (2010) Statistical methods for assessing agreement between two methods of clinical measurement. Int J Nurs Stud 47(8):931–936
Bach M (2001) Electrophysiological approaches for early detection of glaucoma. Eur J Ophthalmol 11:S41–S49
Bach M, Gerling J, Geiger K (1992) Optic atrophy reduces the pattern-electroretinogram for both fine and coarse stimulus patterns. Clin Vis Sci 7(4):327–333
Mashima Y, Oguchi Y (1985) Clinical-study of the pattern electroretinogram in patients with optic-nerve damage. Doc Ophthalmol 61(1):91–96
Thompson DA, Drasdo N (1994) The origins of luminance and pattern responses of the pattern electroretinogram. Int J Psychophysiol 16(2–3):219–227
Ver Hoeve JN et al (1999) VEP and PERG acuity in anesthetized young adult rhesus monkeys. Vis Neurosci 16(4):607–617
Bach M, Hoffmann MB (2008) Update on the pattern electroretinogram in glaucoma. Optom Vis Sci 85(6):386–395
Meigen T, Bach M (1999) On the statistical significance of electrophysiological steady-state responses. Doc Ophthalmol 98(3):207–232
Preiser D et al (2013) Photopic negative response versus pattern electroretinogram in early glaucoma. Invest Ophthalmol Vis Sci 54(2):1182–1191
Miyata K et al (2007) Reduction of oscillatory potentials and photopic negative response in patients with autosomal dominant optic atrophy with OPA1 mutations. Invest Ophthalmol Vis Sci 48(2):820–824
Gotoh Y, Machida S, Tazawa Y (2004) Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol 122(3):341–346
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fisher, A.C., McCulloch, D.L., Borchert, M.S. et al. Comparison of human expert and computer-automated systems using magnitude-squared coherence (MSC) and bootstrap distribution statistics for the interpretation of pattern electroretinograms (PERGs) in infants with optic nerve hypoplasia (ONH). Doc Ophthalmol 131, 25–34 (2015). https://doi.org/10.1007/s10633-015-9493-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-015-9493-y