Abstract
Background
This study aimed to identify the pre-adapting light intensity that generated the maximum separation in the parameters of dark adaptation between participants with early age-related macular degeneration (AMD) and healthy control participants in the minimum recording time.
Methods
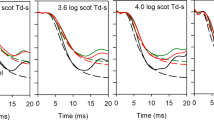
Cone dark adaptation was monitored in 10 participants with early AMD and 10 age-matched controls after exposure to three pre-adapting light intensities, using an achromatic annulus (12° radius) centred on the fovea. Threshold recovery data were modelled, and the time constant of cone recovery (τ), final cone threshold, and time to rod-cone-break (RCB) were determined. The diagnostic potential of these parameters at all pre-adapting intensities was evaluated by constructing receiver operating characteristic (ROC) curves.
Results
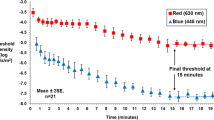
There were significant differences between those with early AMD and healthy controls in cone τ and time to RCB (p < 0.05) at all pre-adapting ‘bleaching’ intensities. ROC curves showed that the diagnostic potential of dark adaptometry was high following exposure to all three pre-adapting intensities, generating an area under the curve in excess of 0.87 ± 0.08 for cone τ and time to RCB for all conditions.
Conclusions
Dark adaptation was shown to be highly diagnostic for early AMD across a range of pre-adapting light intensities, and therefore, the lower pre-adapting intensities evaluated in this study may be used to expedite dark adaptation measurement in the clinic without compromising the integrity of the data obtained. This study reinforces the suggestion that cone and rod dark adaptation are good candidate biomarkers for early AMD.


Similar content being viewed by others
References
Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP (2004) Global data on visual impairment in the year 2002. Bull World Health Organ 82:844–851
Minassian DC, Reidy A, Lightstone A, Desai P (2011) Modelling the prevalence of age-related macular degeneration (2010-2020) in the UK: expected impact of anti-vascular endothelial growth factor (VEGF) therapy. Br J Ophthalmol 95:1433–1436
Owen CG, Jarrar Z, Wormald R, Cook DG, Fletcher AE, Rudnicka AR (2012) The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br J Ophthalmol 96:752–756
Pascolini D, Mariotti SP (2012) Global estimates of visual impairment: 2010. Br J Ophthalmol 96:614–618
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T (2009) Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology 116:57.e55–65.e55
Mitchell P, Korobelnik JF, Lanzetta P, Holz FG, Prunte C, Schmidt-Erfurth U, Tano Y, Wolf S (2010) Ranibizumab (Lucentis) in neovascular age-related macular degeneration: evidence from clinical trials. Br J Ophthalmol 94:2–13
UN (2009) Population prospects: 2008 revision [online]. Available at: http://www.un.org/esa/population/publications/wpp2008/wpp2008_highlights.pdf. Accessed 18 July 2011
Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, de Jong PT, Klaver CC, Klein BEK, Klein R, Mitchell P, Sarks JP, Sarks SH, Soubrane G, Taylor HR, Vingerling JR (1995) An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol 39:367–374
Mei M, Leat SJ (2007) Suprathreshold contrast matching in maculopathy. Invest Ophthalmol Vis Sci 48:3419–3424
Hahn GA, Messias A, MacKeben M, Dietz K, Horwath K, Hyvarinen L, Leinonen M, Trauzettel-Klosinski S (2009) Parafoveal letter recognition at reduced contrast in normal aging and in patients with risk factors for AMD. Graefes Arch Clin Exp Ophthalmol 247:43–51
Sabour-Pickett S, Loughman J, Nolan JM, Stack J, Pesudovs K, Meagher KA, Beatty S (2013) Visual performance in patients with neovascular age-related macular degeneration undergoing treatment with intravitreal ranibizumab. J Ophthal 2013:268438
Eisner A, Stoumbos VD, Klein ML, Fleming SA (1991) Relations between fundus appearance and function—eyes whose fellow eye has exudative age-related macular degeneration. Invest Ophthalmol Vis Sci 32:8–20
Dimitrov PN, Robman LD, Varsamidis M, Aung KZ, Makeyeva GA, Guymer RH, Vingrys AJ (2011) Visual function tests as potential biomarkers in age-related macular degeneration. Invest Ophthalmol Vis Sci 52:9457–9469
Phipps JA, Guymer RH, Vingrys AJ (2003) Loss of cone function in age-related maculopathy. Invest Ophthalmol Vis Sci 44:2277–2283
Mayer MJ, Spiegler SJ, Ward B, Glucs A, Kim CB (1992) Mid-frequency loss of foveal flicker sensitivity in early stages of age-related maculopathy. Invest Ophthalmol Vis Sci 33:3136–3142
Mayer MJ, Spiegler SJ, Ward B, Glucs A, Kim CB (1992) Foveal flicker sensitivity discriminates ARM-risk from healthy eyes. Invest Ophthalmol Vis Sci 33:3143–3149
Mayer MJ, Spiegler SJ, Ward B, Glucs A, Kim CB (1992) Preliminary evaluation of flicker sensitivity as a predictive test for exudative age-related maculopathy. Invest Ophthalmol Vis Sci 33:3150–3155
Rohrschneider K, Bultmann S, Springer C (2008) Use of fundus perimetry (microperimetry) to quantify macular sensitivity. Prog Retin Eye Res 27:536–548
Meleth AD, Mettu P, Agron E, Chew EY, Sadda SR, Ferris FL, Wong WT (2011) Changes in retinal sensitivity in geographic atrophy progression as measured by microperimetry. Invest Ophthalmol Vis Sci 52:1119–1126
Sandberg MA, Gaudio AR (1995) Slow photostress recovery and disease severity in age-related macular degeneration. Retina 15:407–412
Midena E, Angeli CD, Blarzino MC, Valenti M, Segato T (1997) Macular function impairment in eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci 38:469–477
Newsome DA, Negreiro M (2009) Reproducible measurement of macular light flash recovery time using a novel device can indicate the presence and worsening of macular diseases. Curr Eye Res 34:162–170
Owsley C, Jackson GR, White M, Feist R, Edwards D (2001) Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology 108:1196–1202
Owsley C, McGwin G Jr, Jackson GR, Kallies K, Clark M (2007) Cone- and rod-mediated dark adaptation impairment in age-related maculopathy. Ophthalmology 114:1728–1735
Dimitrov PN, Guymer RH, Zele AJ, Anderson AJ, Vingrys AJ (2008) Measuring rod and cone dynamics in age-related maculopathy. Invest Ophthalmol Vis Sci 49:55–65
Gaffney AJ, Binns AM, Margrain TH (2011) The topography of cone dark adaptation deficits in age-related maculopathy. Optom Vis Sci 88:1080–1087
Winsor CP, Clark AB (1936) Dark adaptation after varying degrees of light adaptation. Proc Natl Acad Sci USA 22:400–404
Hecht S, Haig C, Chase AM (1937) The influence of light adaptation on subsequent dark adaptation of the eye. J Gen Physiol 20:831–850
Wald G, Clark AB (1937) Visual adaptation and chemistry of the rods. J Gen Physiol 21:93–105
Haig C (1941) The course of rod dark adaptation as influenced by the intensity and duration of pre-adaptation to light. J Gen Physiol 24:735–751
Mote FA, Riopelle AJ (1951) The effect of varying the intensity and duration of pre-exposure upon foveal dark adaptation in the human eye. J Gen Physiol 34:657–674
Wolf E, Zigler MJ (1954) Location of the break in the dark adaptation curve in relation to pre-exposure brightness and pre-exposure time. J Opt Soc Am 44:875–879
Chylack LT Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, Friend J, McCarthy D, Wu SY (1993) The lens opacities classification system III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol 111:831–836
Metha AB, Vingrys AJ, Badcock DR (1993) Calibration of a color monitor for visual psychophysics. Behav Res Methods Instrum Comput 25:371–383
Brainard DH, Pelli DG, Robson T (2001) Display characterization. In: Hornak J (ed) The encyclopaedia of imaging science and technology, vol 18. Wiley, Hoboken, NJ, pp 172–188
Hollins M, Alpern M (1973) Dark adaptation and visual pigment regeneration in human cones. J Gen Physiol 62:430–447
Thomas MM, Lamb TD (1999) Light adaptation and dark adaptation of human rod photoreceptors measured from the a-wave of the electroretinogram. J Physiol Lond 518(Pt 2):479–496
Jackson GR, Owsley C, McGwin G (1999) Aging and dark adaptation. Vis Res 39:3975–3982
McGwin G Jr, Jackson GR, Owsley C (1999) Using nonlinear regression to estimate parameters of dark adaptation. Behav Res Methods Instrum Comput 31:712–717
Paupoo AA, Mahroo OA, Friedburg C, Lamb TD (2000) Human cone photoreceptor responses measured by the electroretinogram [correction of electroretinogram] a-wave during and after exposure to intense illumination. J Physiol Lond 529(Pt 2):469–482
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36
Hanley JA, McNeil BJ (1983) A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148:839–843
McMurdo MET, Gaskell A (1991) Dark adaptation and falls in the elderly. Gerontology 37:221–224
Gaffney AJ, Binns AM, Margrain TH (2012) Aging and cone dark adaptation. Optom Vis Sci 89:1219–1224
Mahroo OA, Lamb TD (2004) Recovery of the human photopic electroretinogram after bleaching exposures: estimation of pigment regeneration kinetics. J Physiol Lond 554:417–437
Mahroo OAR, Lamb TD (2012) Slowed recovery of human photopic ERG a-wave amplitude following intense bleaches: a slowing of cone pigment regeneration? Doc Ophthalmol 125:137–147
Acknowledgments
This study was funded by a research grant from the College of Optometrists, UK.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaffney, A.J., Binns, A.M. & Margrain, T.H. The effect of pre-adapting light intensity on dark adaptation in early age-related macular degeneration. Doc Ophthalmol 127, 191–199 (2013). https://doi.org/10.1007/s10633-013-9400-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-013-9400-3