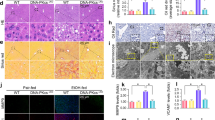
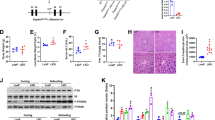
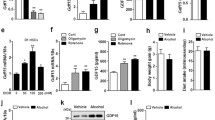
Abstract
Background and Aim
he mixed lineage kinase domain like pseudokinase (MLKL) is known to play a protective role in non-alcoholic fatty liver disease (NAFLD) via inhibition of necroptosis pathway. However, the role of MLKL in alcoholic liver disease (ALD) is not yet clear.
Method
C57BL/6N wild-type (WT) and MLKL-knockout (KO) mice (8–10 weeks old) were randomly divided into eight groups. To establish ALD model of different durations, ethanol (EtOH) was fed to WT and MLKL KO for 10 days, 4 weeks, and 8 weeks. The control group was fed with Lieber-DeCarli control diet for 8 weeks. Mortality, degree of hepatic inflammation, and steatosis were compared among the groups. Bulk mRNA transcriptome analysis was performed. Abundance of transcript and gene expressions were calculated based on read count or Transcript by Million (TPM) value.
Results
Survival rate of MLKL KO mice compared to WT was similar until 4 weeks, but the survival of MLKL KO mice significantly decreased after 8 weeks in ALD model. There was no difference in degree of inflammation, steatosis, and NAS scores between EtOH-fed MLKL KO and EtOH-fed WT mice at 10 days. However, at 4 weeks and 8 weeks, the degree of hepatic steatosis, NAS, and inflammation were increased in MLKL KO mice. RNA transcriptome data showed that fatty acid synthesis, and lipogenesis, mitochondria, and apoptosis-related pathways were upregulated in EtOH-fed MLKL KO mice compared to EtOH-fed WT mice. Although hepatocyte apoptosis (BAX/BCL2 ratio, caspase-3, and TUNEL staining) increased after EtOH intake; however, apoptosis was more significantly increased in EtOH-fed MLKL KO mice compared to the WT group. At the same time, hepatic cFLIP was decreased in EtOH-fed MLKL KO mice compared to the WT group.
Conclusion
MLKL deletion did not prevent chronic alcohol-induced liver damage independently of necroptosis and exacerbated hepatic steatosis by increasing hepatocyte apoptosis.
Graphical Abstract






Similar content being viewed by others
Data availability
Data available on request due to privacy/ethical restrictions.
Abbreviations
- ALD:
-
Alcoholic Liver Disease
- AST:
-
Aspartate Aminotransferase
- ALT:
-
Alanine Aminotransferase
- BAX:
-
Bcl-2-associated X protein
- BCL2:
-
B-Cell Leukemia/Lymphoma Protein-2
- CXCL1:
-
CXC Motif Chemokine Ligand-1
- CXCL2:
-
CXC Motif Chemokine Ligand-2
- c-FLIP:
-
Cellular FLICE Inhibitory Protein
- EtOH:
-
Ethanol/Alcohol
- FAS:
-
Fatty Acid Synthase
- GAPDH:
-
Glyceraldehyde 3-phosphate Dehydrogenase
- JNK:
-
C-Jun N-Terminal Kinase
- p-JNK:
-
Phosphorylated JNK
- RIPK1:
-
Receptor-interacting Protein Kinase 1
- RIPK3:
-
Receptor-interacting Protein Kinase 3
- MLKL:
-
Mixed-Lineage Kinase Domain Like Pseudokinase
- NAFLD:
-
Non-alcoholic Liver Disease
- NAS:
-
Non-Alcoholic Fatty Liver Disease Activity Score
- Ppar-γ:
-
Peroxisome Proliferator Activated Receptor Gamma
- SCD-1:
-
Stearoyl-Coenzyme A Desaturase-1
- SerpinA12:
-
Serpin Peptidase Inhibitor, Clade A Member 12
- SREBP1c:
-
Sterol Regulatory Element-binding Transcription Factor 1
- TG:
-
Triglyceride
- TLR4:
-
Toll-Like Receptor-4
- TLR9:
-
Toll-Like Receptor-9
- TUNEL:
-
Terminal deoxynucleotidyl Transferase dUTP Nick End Labeling
- WT:
-
Wild Type
References
Zhan C, Huang M, Yang X, Hou J. MLKL: functions beyond serving as the executioner of necroptosis. Theranostics 2021;11:4759–4769.
Oh JH, Saeed WK, Kim HY et al. Hepatic stellate cells activate and avoid death under necroptosis stimuli: hepatic fibrosis during necroptosis. J Gastroenterol Hepatol 2023;38:2206–2214.
Oh JH, Park S, Hong E et al. Novel inhibitor of mixed-lineage kinase domain-like protein the antifibrotic effects of a necroptosis antagonist. ACS Pharmacol Transl Sci 2023;6:1471–1479.
Zhou Y, Wu R, Wang X, Bao X, Lu C. Roles of necroptosis in alcoholic liver disease and hepatic pathogenesis. Cell Prolif 2022;55:e13193.
Afonso MB, Rodrigues PM, Mateus-Pinheiro M et al. RIPK3 acts as a lipid metabolism regulator contributing to inflammation and carcinogenesis in non-alcoholic fatty liver disease. Gut 2021;70:2359–2372.
Saeed WK, Jun DW, Jang K et al. Decrease in fat de novo synthesis and chemokine ligand expression in non-alcoholic fatty liver disease caused by inhibition of mixed lineage kinase domain-like pseudokinase. J Gastroenterol Hepatol 2019;34:2206–2218.
Wu X, Poulsen KL, Sanz-Garcia C et al. MLKL-dependent signaling regulates autophagic flux in a murine model of non-alcohol-associated fatty liver and steatohepatitis. J Hepatol 2020;73:616–627.
Tonon M, Piano S. Alcohol-related cirrhosis: the most challenging etiology of cirrhosis is more burdensome than ever. Clin Mol Hepatol 2021;27:94–96.
Saeed WK, Jun DW, Jang K, Koh DH. Necroptosis signaling in liver diseases: an update. Pharmacol Res 2019;148:104439.
Saeed WK, Jun DW, Jang K et al. Mismatched effects of receptor interacting protein kinase-3 on hepatic steatosis and inflammation in non-alcoholic fatty liver disease. World J Gastroenterol 2018;24:5477–5490.
Zhang Z, Xie G, Liang L et al. RIPK3-mediated necroptosis and neutrophil infiltration are associated with poor prognosis in patients with alcoholic cirrhosis. J Immunol Res 2018;2018:1509851.
Miyata T, Wu X, Fan X et al. Differential role of MLKL in alcohol-associated and non-alcohol-associated fatty liver diseases in mice and humans. JCI Insight. 2021. https://doi.org/10.1172/jci.insight.140180.
Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology 2013;57:1773–1783.
Chen H, McKeen T, Chao X et al. The role of MLKL in Hepatic ischemia-reperfusion injury of alcoholic steatotic livers. Int J Biol Sci 2022;18:1096–1106.
Wu X, Fan X, McMullen MR et al. Macrophage-derived MLKL in alcohol-associated liver disease: regulation of phagocytosis. Hepatology. 2022. https://doi.org/10.1016/S0168-8278(22)00478-0.
Miyata T, Nagy LE. Programmed cell death in alcohol-associated liver disease. Clin Mol Hepatol 2020;26:618–625.
Wu J, Huang Z, Ren J, Zhang Z, He P, Li Y, Ma J et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res 2013;23:994–1006.
Zhu Q, Han X, Peng J, Qin H, Wang Y. The role of CXC chemokines and their receptors in the progression and treatment of tumors. J Mol Histol 2012;43:699–713.
Wang K. Molecular mechanisms of hepatic apoptosis regulated by nuclear factors. Cell Signal 2015;27:729–738.
Suda J, Dara L, Yang L, Aghajan M, Song Y, Kaplowitz N, Liu ZX. Knockdown of RIPK1 markedly exacerbates murine immune-mediated liver injury through massive apoptosis of hepatocytes, independent of necroptosis and inhibition of NF-kappaB. J Immunol 2016;197:3120–3129.
Funding
This research was supported by Korea Drug Development Fund funded by Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (RS-2021-DD121073, Republic of Korea). And This study was supported by The Research Supporting Program of The Korean Association for the Study of the Liver (KASL2019-04).
Author information
Authors and Affiliations
Contributions
Guarantor of the article: Dae Won Jun, Concept and design: Eileen L. Yoon, Data collection and management: Keon Hwi Im, Xuanyuan hanning, Eun Bin Kim, A Hyeon Lee, Ji Eun Kim, Histology interpretation: Hyun Sung Kim, Interpretation of data: Seung Min Lee, Writing of the manuscript: Waqar Khalid Saeed, and Keon Hwi Im, All authors have approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has any potential financial conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Im, K.H., Saeed, W.K., Kim, E.B. et al. Deletion of Mixed Lineage Kinase Domain Like Pseudokinase Aggravates Chronic Alcohol-Induced Liver Injury via Increasing Apoptosis. Dig Dis Sci (2024). https://doi.org/10.1007/s10620-024-08310-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10620-024-08310-2