Abstract
Background
Hepatic stellate cell hyperactivation is a central link in liver fibrosis development, transforming growth factor β1 (TGF-β1) is a key activator of HSCs.
Aims
This study investigated whether anlotinib attenuates CCl4 induced liver fibrosis in mice and explored its antifibrotic mechanism.
Methods
We used the human hepatic stellate cell line LX-2 for in vitro assays and used TGF-β1 to induce hepatic fibrosis in LX-2 cells. We analyzed cytotoxicity using a cell-counting kit-8 and transwell chambers to detect the migratory ability of LX-2 cells. Western blotting was used to detect the protein levels of collagen type I, α-smooth muscle actin, and p-Smad3. In addition, mice with CCl4-induced hepatic fibrosis were used as in vivo models. Histopathological examination was performed using H&E staining, Masson’s trichrome staining, and immunohistochemistry.
Results
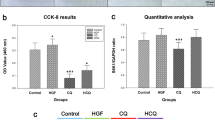
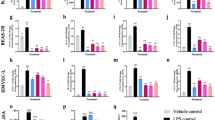
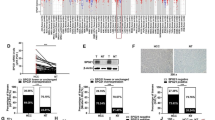
Anlotinib significantly reversed TGF-β1-induced protein levels of Col I, α-SMA and p-Smad3 and inhibits migratory and proliferative abilities in vitro using LX-2 cells. CCl4 cause F4 grade (Ishak) hepatic fibrosis, liver inflammatory scores ranged from 12 to 14 (Ishak), a mean ALT measurement of 130 U/L and a mean measurement AST value of 119 U/L in mice. However, the CCl4-induced changes were markedly attenuated by anlotinib treatment, which returned to F2 grade (Ishak) hepatic fibrosis, liver inflammatory scores ranged from 4 to 6 (Ishak), a mean ALT measurement of 40 U/L and a mean measurement AST value of 56 U/L in mice.
Conclusions
Our results suggest that anlotinib-mediated suppression of liver fibrosis is related to the inhibition of TGF-β1 signaling pathway.
Graphical Abstract
Hepatic stellate cell hyper activation is a central link in liver fibrosis development, transforming growth factor β1 is a key activator of HSCs. Anlotinib is a multi-targeted tyrosine kinase inhibitor that has similar targets to nintedanib, a clinically used anti-pulmonary fibrosis drug. Our study demonstrates an FDA-approved drug—anlotinib—that could prevent liver fibrosis and inflammation. Experiments in cell cultures and mice show that anlotinib can inhibit the activation of hepatic stellate cells by down-regulating the TGFβ1/smad3 pathway, thereby reversing liver fibrosis. In animal experiments, anlotinib showed protective effects on the CCl4-induced liver damage, including ameliorating liver inflammation, reversing liver fibrosis and reducing liver enzymes. This is a very good signal, anlotinib may be useful for halting or reversing the progression of liver fibrosis and could be employed in the development of novel therapeutic drugs for the management of chronic liver diseases.





Similar content being viewed by others
Data Availability
All raw data supporting our findings is available on request.
References
Adenina S, Louisa M, Soetikno V, Arozal W, Wanandi SI. The effect of alpha mangostin on epithelial-mesenchymal transition on human hepatocellular carcinoma HepG2 cells surviving Sorafenib via TGF-beta/Smad pathways. Adv Pharm Bull 2020;10:648–655.
Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol 2019;70:151–171.
Brenner DA. Reversibility of liver fibrosis. Gastroenterol Hepatol 2013;9:737–739.
Cai S, Wu L, Yuan S, Liu G, Wang Y, Fang L, Xu D. Carvacrol alleviates liver fibrosis by inhibiting TRPM7 and modulating the MAPK signaling pathway. Eur J Pharmacol 2021;898:173982.
Campana L, Iredale JP. Regression of liver fibrosis. Semin Liver Dis 2017;37:1–10.
Chen W, Zhang J, Zhong W, Liu Y, Lu Y, Zeng Z, Huang H, Wan X, Meng X, Zou F, Cai S, Dong H. Anlotinib inhibits PFKFB3-driven glycolysis in myofibroblasts to reverse pulmonary fibrosis. Front Pharmacol 2021;12:744826.
Friedman SL. Liver fibrosis—from bench to bedside. J Hepatol 2003;38:S38–S53.
He C, Wu T, Hao Y. Anlotinib induces hepatocellular carcinoma apoptosis and inhibits proliferation via Erk and Akt pathway. Biochem Bioph Res Co 2018;503:3093–3099.
Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliver Rev 2017;121:27–42.
Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Zhou J, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu L, Chen G, Li H, Huang X, Zhang BH, Yuan Y. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology 2020;72:389–398.
Lei J, Li Q, Xu H, Luo M, Liu Z, Xiang D, Chen P. Anlotinib improves bile duct ligature-induced liver fibrosis in rats via antiangiogenesis regulated by VEGFR2/mTOR pathway. Drug Develop Res. 2022;84:143–155.
Liang L, Hui K, Hu C, Wen Y, Jiang X. Autophagy inhibition potentiates the anti-angiogenic property of multikinase inhibitor anlotinib through JAK2/STAT3/VEGFA signaling in non-small cell lung cancer cells. Ann Oncol 2019;30:ii4.
Liu F, Bayliss G, Zhuang S. Application of nintedanib and other potential anti-fibrotic agents in fibrotic diseases. Clin Sci 2019;133:1309–1320.
Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A. Hepatic fibrosis: molecular mechanisms and drug targets. Annu Rev Pharmacol 2005;45:605–628.
Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, Swigris JJ, Taniguchi H, Wells AU. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers 2017;3:17074.
Marti-Rodrigo A, Alegre F, Moragrega AB, Garcia-Garcia F, Marti-Rodrigo P, Fernandez-Iglesias A, Gracia-Sancho J, Apostolova N, Esplugues JV, Blas-Garcia A. Rilpivirine attenuates liver fibrosis through selective STAT1-mediated apoptosis in hepatic stellate cells. Gut 2020;69:920–932.
Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis 2001;21:397–416.
Raja S, Ahamed KF, Kumar V, Mukherjee K, Bandyopadhyay A, Mukherjee PK. Antioxidant effect of Cytisus scoparius against carbon tetrachloride treated liver injury in rats. J Ethnopharmacol 2007;109:41–47.
Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. New Engl J Med 2014;370:2071–2082.
Ruan H, Lv Z, Liu S, Zhang L, Huang K, Gao S, Gan W, Liu X, Zhang S, Helian K, Li X, Zhou H, Yang C. Anlotinib attenuated bleomycin-induced pulmonary fibrosis via the TGF-beta1 signalling pathway. J Pharm Pharmacol 2020;72:44–55.
Spagnolo P, Kropski JA, Jones MG, Lee JS, Rossi G, Karampitsakos T, Maher TM, Tzouvelekis A, Ryerson CJ. Idiopathic pulmonary fibrosis: disease mechanisms and drug development. Pharmacol Therapeut 2021;222:107798.
Sun M, Kisseleva T. Reversibility of liver fibrosis. Clin Res Hepatol Gas 2015;39:S60–S63.
Sun Y, Niu W, Du F, Du C, Li S, Wang J, Li L, Wang F, Hao Y, Li C, Chi Y. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol 2016;9:105.
Syed YY. Anlotinib: first global approval. Drugs 2018;78:1057–1062.
Taurin S, Yang CH, Reyes M, Cho S, Coombs DM, Jarboe EA, Werner TL, Peterson CM, Janat-Amsbury MM. Endometrial cancers harboring mutated fibroblast growth factor receptor 2 protein are successfully treated with a new small tyrosine kinase inhibitor in an orthotopic mouse model. Int J Gynecol Cancer 2018;28:152–160.
Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastro Hepat 2017;14:397–411.
Unsal V, Cicek M, Sabancilar I. Toxicity of carbon tetrachloride, free radicals and role of antioxidants. Rev Environ Health 2021;36:279–295.
Vallee A, Lecarpentier Y, Vallee JN. Thermodynamic aspects and reprogramming cellular energy metabolism during the fibrosis process. Int J Mol Sci 2017;18:2537.
Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther 2014;349:209–220.
Xia X, Pi W, Lan Y, Wu X, Lv D, Meng Y, Yang H, Wang W. Synergistic antitumor effects of anlotinib combined with oral 5-fluorouracil/S-1 via inhibiting Src/AKT signaling pathway in small-cell lung cancer. Anal Cell Pathol 2022;2022:4484211.
Xie C, Wan X, Quan H, Zheng M, Fu L, Li Y, Lou L. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci 2018;109:1207–1219.
Yang YR, Bu FT, Yang Y, Li H, Huang C, Meng XM, Zhang L, Lv XW, Li J. LEFTY2 alleviates hepatic stellate cell activation and liver fibrosis by regulating the TGF-beta1/Smad3 pathway. Mol Immunol 2020;126:31–39.
Yao Q, Lin Y, Li X, Shen X, Wang J, Tu C. Curcumin ameliorates intrahepatic angiogenesis and capillarization of the sinusoids in carbon tetrachloride-induced rat liver fibrosis. Toxicol Lett 2013;222:72–82.
Yuan M, Guo XL, Chen JH, He Y, Liu ZQ, Zhang HP, Ren J, Xu Q. Anlotinib suppresses proliferation, migration, and immune escape of gastric cancer cells by activating the cGAS-STING/IFN-beta pathway. Neoplasma 2022;69:807–819.
Zhan L, Huang C, Meng XM, Song Y, Wu XQ, Yang Y, Li J. Hypoxia-inducible factor-1alpha in hepatic fibrosis: a promising therapeutic target. Biochimie 2015;108:1–7.
Zhao XK, Yu L, Cheng ML, Che P, Lu YY, Zhang Q, Mu M, Li H, Zhu LL, Zhu JJ, Hu M, Li P, Liang YD, Luo XH, Cheng YJ, Xu ZX, Ding Q. Focal adhesion kinase regulates hepatic stellate cell activation and liver fibrosis. Sci Rep-UK 2017;7:4032.
Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroentero 2014;20:7312–7324.
Acknowledgments
This work was supported by the Science and Technology Fund Project of Guizhou Provincial Health Commission (contract numbers gzwkj2021-074 to Ye-Ting Wu) , the National Natural Science Foundation of China (grant numbers 82060116 and 82260129 to Xue-Ke Zhao) and the Science and Technology Support Project of Guizhou Province, No. [2021] 094. We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This research was funded by Science and Technology Fund Project of Guizhou Provincial Health Commission (contract numbers gzwkj2021-074 to Ye-Ting Wu), the National Natural Science Foundation of China (grant numbers 82060116 and 82260129 to Xue-Ke Zhao) and the Science and Technology Support Project of Guizhou Province(No. [2021] 094).
Author information
Authors and Affiliations
Contributions
Conceptualization: XKZ, WFZ. Data curation: YTW, QZL, XKZ, MM, GLZ, WFZ. Formal analysis: YTW, QZL. Methodology and Resources: YTW, XKZ, WFZ. Project administration and Writing – original draft: YTW. Software: QZL. Supervision: WFZ. Visualization: YTW, WFZ. Writing – review & editing: YTW, QZL, XKZ.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest in this work.
Ethics approval
All experiments involving animals should obtain relevant ethics approval in accordance with national and institutional guidelines prior to commencing any animal work. This study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the ethics committee of Guizhou Medical University, Guizhou, China. The animal experimental ethical number is 2000732.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, YT., Li, QZ., Zhao, XK. et al. Anlotinib Attenuates Liver Fibrosis by Regulating the Transforming Growth Factor β1/Smad3 Signaling Pathway. Dig Dis Sci 68, 4186–4195 (2023). https://doi.org/10.1007/s10620-023-08101-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08101-1