Abstract
Background
Percutaneous liver biopsy (P-bx) is the gold standard for diagnosing advanced fibrosis. Despite the proven technical feasibility of EUS-guided liver bx (EUS-bx) as a novel alternative way of liver biopsy, the clinical applicability remains to be determined.
Aims
The primary aim of this study is to evaluate if EUS-bx, compared to P-bx, can effectively and safely obtain adequate specimen and accurately predict hepatic fibrosis.
Methods
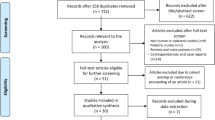
This is a single center, retrospective chart review among patients with liver diseases at a tertiary endoscopy center from February 2011 to March 2020. We assessed the EUS-bx versus P-bx outcomes by success rate, performance, and safety profile. The primary outcome was the association between EUS-bx clinical variables and the presence of histologic liver fibrosis stage ≥ 3. The secondary outcomes were the associations between EUS-bx and variables indicative of fibrosis.
Results
Fifty-nine patients underwent EUS-bx; and 59, P-bx. All EUS-bx procedures were successfully completed. All 56/56 (100%) of EUS-bx vs. 50/52 (96.2%) P-bx were considered adequate samples. Tissue lengths were significantly longer in the EUS-bx cohort (p < 0.0001) with a trend towards a greater number of portal tracts. 46/56 (82.1%) cases of EUS-bx vs. 32/52 (61.5%) of P-bx had > 10 portal tracts; 21/56 (37.5%) cases of EUS-bx vs. 14/52 (26.9%) of P-bx had > 15 portal tracts. There were 6 (10.2%) EUS-bx vs. 1 (1.7%) P-bx related complication leading to a phone call (p = 0.061).
Conclusions
EUS-bx can safely performed and accurately predict liver fibrosis stage as the standard P-bx without being influenced by procedure-related factors.
Similar content being viewed by others
Abbreviations
- HVPG:
-
Hepatic venous pressure gradient
- EUS-PPG:
-
EUS-guided portal pressure gradient measurement
- EUS-bx:
-
EUS-guided liver biopsy
- EV:
-
Esophageal varices
- GV:
-
Gastric varices
- PHG:
-
Portal hypertensive gastropathy
- CTP:
-
Child-Turcotte Pugh
- MELD:
-
Model for end-stage liver disease
- APRI:
-
AST to platelet ratio index
- FIB-4:
-
Fibrosis-4
References
Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017–1044. https://doi.org/10.1002/hep.22742.
Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:475–485. https://doi.org/10.3748/wjg.v20.i2.475.
Huang JY, Samarasena JB, Tsujino T, Chang KJ. EUS-guided portal pressure gradient measurement with a novel 25-gauge needle device versus standard transjugular approach: a comparison animal study. Gastrointest Endosc. 2016;84:358–362. https://doi.org/10.1016/j.gie.2016.02.032.
Huang JY, Samarasena JB, Tsujino T et al. EUS-guided portal pressure gradient measurement with a simple novel device: a human pilot study. Gastrointest Endosc. 2017;85:996–1001. https://doi.org/10.1016/j.gie.2016.09.026.
Samarasena JB, Huang JY, Tsujino T et al. EUS-guided portal pressure gradient measurement with a simple novel device: a human pilot study. VideoGIE. 2018;3:361–363. https://doi.org/10.1016/j.vgie.2018.07.013.
Samarasena JB, Chang KJ. Endoscopic Ultrasound-Guided Portal Pressure Measurement and Interventions. Clin Endosc. 2018;51:222–228. https://doi.org/10.5946/ce.2018.079.
Mansour D, McPherson S. Management of decompensated cirrhosis. Clin Med. 2018;18:s60–s65. https://doi.org/10.7861/clinmedicine.18-2-s60.
Lin Z-H, Xin Y-N, Dong Q-J et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: An updated meta-analysis. Hepatology. 2011;53:726–736. https://doi.org/10.1002/hep.24105.
Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi:https://doi.org/10.1002/hep.21669
Knodell RG, Ishak KG, Black WC et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. https://doi.org/10.1002/hep.1840010511.
Bazerbachi F, Vargas EJ, Matar R et al. EUS-guided core liver biopsy sampling using a 22-gauge fork-tip needle: a prospective blinded trial for histologic and lipidomic evaluation in nonalcoholic fatty liver disease. Gastrointest Endosc. 2019;90:926–932. https://doi.org/10.1016/j.gie.2019.08.006.
Mohan BP, Shakhatreh M, Garg R, Ponnada S, Adler DG. Efficacy and safety of EUS-guided liver biopsy: a systematic review and meta-analysis. Gastrointest Endosc. 2019;89:238-246.e3. https://doi.org/10.1016/j.gie.2018.10.018.
Sey MSL, Al-Haddad M, Imperiale TF, McGreevy K, Lin J, DeWitt JM. EUS-guided liver biopsy for parenchymal disease: a comparison of diagnostic yield between two core biopsy needles. Gastrointest Endosc. 2016;83:347–352. https://doi.org/10.1016/j.gie.2015.08.012.
Funding
This was an investigator initiated study, and there was no funding for this study.
Author information
Authors and Affiliations
Contributions
AYC: data collection, manuscript preparation and review. XL: review of pathology. WG: review of pathology, manuscript review. VC: review of pathology, manuscript review. JS: procedure performed, manuscript review. JL: procedure performed, manuscript review. KC: procedure performed, manuscript review. K-QH: Study design, statistical analysis, manuscript preparation and review.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest to disclose related to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An editorial commenting on this article is available at https://doi.org/10.1007/s10620-023-08020-1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choi, A.Y., Li, X., Guo, W. et al. Performance of Endoscopic Ultrasound-Guided Versus Percutaneous Liver Biopsy in Diagnosing Stage 3–4 Fibrosis. Dig Dis Sci 68, 3774–3780 (2023). https://doi.org/10.1007/s10620-023-08019-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08019-8