Abstract
Background
Pancreatic ductal adenocarcinoma (PDA) has a poor prognosis due to its therapeutic resistance. Inactivation of vitamin D/vitamin D receptor (VDR) signaling may contribute to the malignant phenotype of PDA and altered expression of oncoprotein mucin 1 (MUC1) may be involved in drug resistance of cancer cells.
Aim
To determine whether vitamin D/VDR signaling regulates the expression and function of MUC1 and its effect on acquired gemcitabine resistance of pancreatic cancer cells.
Methods
Molecular analyses and animal models were used to determine the impact of vitamin D/VDR signaling on MUC1 expression and response to gemcitabine treatment.
Results
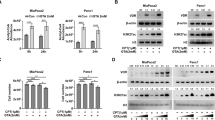
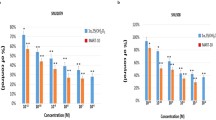
RPPA analysis indicated that MUC1 protein expression was significantly reduced in human PDA cells after treatment with vitamin D3 or its analog calcipotriol. VDR regulated MUC1 expression in both gain- and loss-of-function assays. Vitamin D3 or calcipotriol significantly induced VDR and inhibited MUC1 expression in acquired gemcitabine-resistant PDA cells and sensitized the resistant cells to gemcitabine treatment, while siRNA inhibition of MUC1 was associated with paricalcitol-associated sensitization of PDA cells to gemcitabine treatment in vitro. Administration of paricalcitol significantly enhanced the therapeutic efficacy of gemcitabine in xenograft and orthotopic mouse models and increased the intratumoral concentration of dFdCTP, the active metabolite of gemcitabine.
Conclusion
These findings demonstrate a previously unidentified vitamin D/VDR-MUC1 signaling axis involved in the regulation of gemcitabine resistance in PDA and suggests that combinational therapies that include targeted activation of vitamin D/VDR signaling may improve the outcomes of patients with PDA.






Similar content being viewed by others
Data Availability
The raw protein RPPA data generated in this study are available in TCPA database under accession number #TCPA00000010 (with link: http://tcpaportal.org/tcpa/download/TCPA00000010.zip). Other data that support the findings of this study are available from the corresponding author upon request.
Abbreviations
- MUC1:
-
Mucin 1
- PDA:
-
Pancreatic ductal adenocarcinoma
- PanIN:
-
Pancreatic intraepithelial neoplasia
- VDR:
-
Vitamin D receptor
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33.
Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet (London, England) 2020;395:2008–2020.
Zeng S, Pöttler M, Lan B, Grützmann R, Pilarsky C, Yang H. Chemoresistance in pancreatic cancer. Int J Mol Sci 2019;20:4504.
Amrutkar M, Gladhaug IP. Pancreatic cancer chemoresistance to gemcitabine. Cancers 2017;9:157.
Burris HA 3rd, Moore MJ, Andersen J et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol Off J Am Soc Clin Oncol 1997;15:2403–2413.
Tsujimoto A, Sudo K, Nakamura K et al. Gemcitabine plus nab-paclitaxel for locally advanced or borderline resectable pancreatic cancer. Sci Rep 2019;9:16187.
Neesse A, Michl P, Frese KK et al. Stromal biology and therapy in pancreatic cancer. Gut 2011;60:861–868.
Wei D, Wang L, Zuo X, Bresalier RS. Vitamin D: promises on the horizon and challenges ahead for fighting pancreatic cancer. Cancers 2021;13:2716.
Cho M, Peddi PF, Ding K et al. Vitamin D deficiency and prognostics among patients with pancreatic adenocarcinoma. J Transl Med 2013;11:206.
Innocenti F, Owzar K, Jiang C et al. The vitamin D receptor gene as a determinant of survival in pancreatic cancer patients: genomic analysis and experimental validation. PLoS ONE 2018;13:e0202272.
Yuan C, Qian ZR, Babic A et al. Prediagnostic Plasma 25-hydroxyvitamin D and pancreatic cancer survival. J Clin Oncol Off J Am Soc Clin Oncol 2016;34:2899–2905.
Sherman MH, Yu RT, Engle DD et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014;159:80–93.
Anbil S, Pigula M, Huang HC et al. Vitamin D receptor activation and photodynamic priming enables durable low-dose chemotherapy. Mol Cancer Ther 2020;19:1308–1319.
Li Z, Jia Z, Gao Y et al. Activation of vitamin D receptor signaling downregulates the expression of nuclear FOXM1 protein and suppresses pancreatic cancer cell stemness. Clin Cancer Res Off J Am Assoc Cancer Res 2015;21:844–853.
Kufe DW. MUC1-C in chronic inflammation and carcinogenesis; emergence as a target for cancer treatment. Carcinogenesis 2020;41:1173–1183.
Nath S, Mukherjee P. MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med 2014;20:332–342.
Nagata K, Horinouchi M, Saitou M et al. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepato-Biliary-Pancreat Surg 2007;14:243–254.
Hinoda Y, Ikematsu Y, Horinochi M et al. Increased expression of MUC1 in advanced pancreatic cancer. J Gastroenterol 2003;38:1162–1166.
Rachagani S, Torres MP, Kumar S et al. Mucin (Muc) expression during pancreatic cancer progression in spontaneous mouse model: potential implications for diagnosis and therapy. J Hematol Oncol 2012;5:68.
Tinder TL, Subramani DB, Basu GD et al. MUC1 enhances tumor progression and contributes toward immunosuppression in a mouse model of spontaneous pancreatic adenocarcinoma. J Immunol (Baltimore, Md: 1950) 2008;181:3116–3125.
Besmer DM, Curry JM, Roy LD et al. Pancreatic ductal adenocarcinoma mice lacking mucin 1 have a profound defect in tumor growth and metastasis. Cancer Res 2011;71:4432–4442.
Nath S, Daneshvar K, Roy LD et al. MUC1 induces drug resistance in pancreatic cancer cells via upregulation of multidrug resistance genes. Oncogenesis 2013;2:e51.
Ren J, Agata N, Chen D et al. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell 2004;5:163–175.
Shukla SK, Purohit V, Mehla K et al. MUC1 and HIF-1alpha signaling crosstalk induces anabolic glucose metabolism to impart gemcitabine resistance to pancreatic cancer. Cancer Cell 2017;32:71–87.
Huang C, Qiu Z, Wang L et al. A novel FoxM1-caveolin signaling pathway promotes pancreatic cancer invasion and metastasis. Cancer Res 2012;72:655–665.
Li J, Akbani R, Zhao W et al. Explore, visualize, and analyze functional cancer proteomic data using the cancer proteome atlas. Cancer Res 2017;77:e51–e54.
Wei D, Kanai M, Jia Z, Le X, Xie K. Kruppel-like factor 4 induces p27Kip1 expression in and suppresses the growth and metastasis of human pancreatic cancer cells. Cancer Res 2008;68:4631–4639.
Yan Y, Li Z, Kong X et al. KLF4-mediated suppression of CD44 signaling negatively impacts pancreatic cancer stemness and metastasis. Cancer Res 2016;76:2419–2431.
Li L, Long J, Mise K et al. PGC1α is required for the renoprotective effect of lncRNA Tug1 in vivo and links Tug1 with urea cycle metabolites. Cell Rep 2021;36:109510.
Naveh-Many T, Silver J. Effects of calcitriol, 22-oxacalcitriol, and calcipotriol on serum calcium and parathyroid hormone gene expression. Endocrinology 1993;133:2724–2728.
Cheng J, Zhang W, Zhang X, Li X, Chen J. Efficacy and safety of paricalcitol therapy for chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol CJASN 2012;7:391–400.
Yan Y, Zuo X, Wei D. Concise review: emerging role of CD44 in cancer stem cells: a promising biomarker and therapeutic target. Stem Cells Transl Med 2015;4:1033–1043.
Santana-Codina N, Roeth AA, Zhang Y et al. Oncogenic KRAS supports pancreatic cancer through regulation of nucleotide synthesis. Nat Commun 2018;9:4945.
Ying H, Kimmelman AC, Lyssiotis CA et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012;149:656–670.
Rasmussen LS, Yilmaz MK, Falkmer UG et al. Pre-treatment serum vitamin D deficiency is associated with increased inflammatory biomarkers and short overall survival in patients with pancreatic cancer. Eur J Cancer (Oxford, England: 1990) 2021;144:72–80.
Fernández-Barral A, Costales-Carrera A, Buira SP et al. Vitamin D differentially regulates colon stem cells in patient-derived normal and tumor organoids. FEBS J 2020;287:53–72.
Hata T, Rajabi H, Takahashi H et al. MUC1-C activates the NuRD complex to drive dedifferentiation of triple-negative breast cancer cells. Cancer Res 2019;79:5711–5722.
Le Large TYS, El Hassouni B, Funel N et al. Proteomic analysis of gemcitabine-resistant pancreatic cancer cells reveals that microtubule-associated protein 2 upregulation associates with taxane treatment. Ther Adv Med Oncol 2019;11:1758835919841233.
Binenbaum Y, Na’ara S, Gil Z. Gemcitabine resistance in pancreatic ductal adenocarcinoma. Drug Resistance Updates Rev Commentaries Antimicrobial Anticancer Chemother 2015;23:55–68.
Ko AH, LoConte N, Tempero MA et al. A Phase I Study of FOLFIRINOX Plus IPI-926, a Hedgehog Pathway Inhibitor, for Advanced Pancreatic Adenocarcinoma. Pancreas 2016;45:370–375.
Van Cutsem E, Tempero MA, Sigal D et al. Randomized phase III trial of pegvorhyaluronidase alfa with nab-paclitaxel plus gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. J Clin Oncol Off J Am Soc Clin Oncol 2020;38:3185–3194.
Olive KP, Jacobetz MA, Davidson CJ et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science (New York, NY) 2009;324:1457–1461.
Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21:418–429.
Santiso-Mere D, Sone T, Hilliard GM, Pike JW, McDonnell DP. Positive regulation of the vitamin D receptor by its cognate ligand in heterologous expression systems. Mol Endocrinol (Baltimore, Md) 1993;7:833–839.
Miller CW, Morosetti R, Campbell MJ, Mendoza S, Koeffler HP. Integrity of the 1,25-dihydroxyvitamin D3 receptor in bone, lung, and other cancers. Mol Carcinogenes 1997;19:254–257.
Roy LD, Sahraei M, Subramani DB et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene 2011;30:1449–1459.
Sahraei M, Bose M, Sanders JA et al. Repression of MUC1 promotes expansion and suppressive function of myeloid-derived suppressor cells in pancreatic and breast cancer murine models. Int J Mol Sci 2021;22:5587.
Tsutsumida H, Swanson BJ, Singh PK et al. RNA interference suppression of MUC1 reduces the growth rate and metastatic phenotype of human pancreatic cancer cells. Clin Cancer Res Off J Am Assoc Cancer Res 2006;12:2976–2987.
Chaika NV, Gebregiworgis T, Lewallen ME et al. MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proc Natl Acad Sci USA 2012;109:13787–13792.
Olou AA, King RJ, Yu F, Singh PK. MUC1 oncoprotein mitigates ER stress via CDA-mediated reprogramming of pyrimidine metabolism. Oncogene 2020;39:3381–3395.
Nagai K, Adachi T, Harada H, Eguchi S, Sugiyama H, Miyazaki Y. Dendritic cell-based immunotherapy pulsed with wilms tumor 1 peptide and mucin 1 as an adjuvant therapy for pancreatic ductal adenocarcinoma after curative resection: a phase i/iia clinical trial. Anticancer Res 2020;40:5765–5776.
Striefler JK, Riess H, Lohneis P et al. Mucin-1 protein is a prognostic marker for pancreatic ductal adenocarcinoma: results from the CONKO-001 study. Front Oncol 2021;11:670396.
Wang S, You L, Dai M, Zhao Y. Quantitative assessment of the diagnostic role of mucin family members in pancreatic cancer: a meta-analysis. Ann Transl Med 2021;9:192.
Acknowledgments
This study made use of the MD Anderson Cancer Center Functional Proteomics RPPA Core Facility, the Metabolomics Core Facility, and Research Animal Support Facility, supported by Cancer Center Support Grant CA016672. We thank Dawn Chalaire, scientific editor in the Research Medical Library at MD Anderson Cancer Center, for editing the manuscript. The authors thank Wei Li for the graphic arts used in the manuscript.
Funding
The work was supported by the National Cancer Institute (R01 CA198090 to R.S.B), the NIH-funded Texas Medical Center Digestive Disease Center Research Core Center Program P30DK056338 (to R.S.B and D.W), and grants from the Elsa U. Pardee Foundation, the University of Texas MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment, and the MD Anderson Cancer Center Institutional Research (to D.W).
Author information
Authors and Affiliations
Contributions
DW and RB conceived the study, designed the experiments, and wrote the manuscript. DW, LW, YL, XZ, and MH performed various portions of animal experiments and histologic analysis and the in vitro experiments. YL performed part of the bioinformatic data analysis. PY, LT, and PLL helped with IC-MS assay and data analysis. XZ provided conceptual feedback for the manuscript. All authors have read and agreed to the publication of the manuscript. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no disclosures.
Ethical approval
The animal protocol and human subject protocol involved in this study were approved by the institutional Review Board of UT MD Anderson Cancer Center.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, D., Wang, L., Liu, Y. et al. Activation of Vitamin D/VDR Signaling Reverses Gemcitabine Resistance of Pancreatic Cancer Cells Through Inhibition of MUC1 Expression. Dig Dis Sci 68, 3043–3058 (2023). https://doi.org/10.1007/s10620-023-07931-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-07931-3