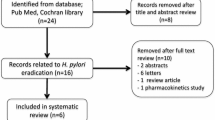
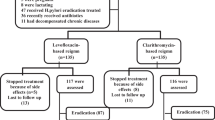
Abstract
Introduction
Helicobacter pylori infects a large percentage of the world’s population and is etiologically related to gastric cancer. The U.S. Food and Drug Administration recently approved two 14-day vonoprazan-containing regimens (vonoprazan–amoxicillin with or without clarithromycin) for H. pylori infections in the United States/Europe.
Methods
We critically reviewed the trial methods to discover why the results were unacceptable low [i.e., no regimen achieved clinically acceptable (≥ 90%) or even conditionally acceptable cure rates (≥ 85%)]. Cure rates with antibiotic susceptible strains were 84.7 for vonoprazan triple therapy, 78.5 for vonoprazan–amoxicillin, and 78.7 for lansoprazole triple therapy, respectively. As was previously shown in Japan, the benefit from adding clarithromycin to vonoprazan–amoxicillin was minimal and the majority of the clarithromycin administered was unnecessary.
Results
The possible reasons for failure to achieve high cure rates discussed include (a) reduced intragastric antibiotic concentrations, (b) an increase in heteroresistance, and (c) failure to achieve an intragastric pH conducive for amoxicillin to eradicate the infection. In addition, there was no pilot study or other attempt to optimize any regimen.
Conclusion
The most likely reason for failure was failure to achieve high intragastric concentrations of antibiotics or to achieve an intragastric pH conducive for amoxicillin to be active. Importantly, vonoprazan triple therapy resulted in > 10 tons of unneeded clarithromycin/million courses of vonoprazan triple therapy. Antibiotic misuse combined with low cure rates suggest that vonoprazan–clarithromycin triple therapies should not be prescribed for H. pylori infection. Dual vonoprazan–amoxicillin therapy has proven effective elsewhere and after optimization may eventually prove useful in the U.S./Europe.



Similar content being viewed by others
References
FDA. Vonoprazan Package Insert, 2022. Available at: http://www.phathompharma.com/wp-content/uploads/VOQUEZNA-TRIPLE-PAK-and-VOQUEZNA-DUAL-PAK-FDA-Final-Label-3.pdf#page=38. Accessed 6/10/2022, 2022.
Chey WD, Mégraud F, Laine L, López LJ, Hunt BJ, Howden CW. Vonoprazan Triple and Dual Therapy for Helicobacter pylori Infection in the US and Europe: Randomized Clinical Trial Gastroenterology. 2022.
Graham DY, Liou JM. Primer for Development of Guidelines for Helicobacter pylori Therapy Using Antimicrobial Stewardship. Clin Gastroenterol Hepatol. 2022;20:e971.
Lee YC, Dore MP, Graham DY. Diagnosis and Treatment of Helicobacter pylori Infection. Annu Rev Med. 2022;73:183–195.
Tanabe H, Yoshino K, Ando K et al. Vonoprazan-based triple therapy is non-inferior to susceptibility-guided proton pump inhibitor-based triple therapy for Helicobacter pylori eradication. Ann Clin Microbiol Antimicrob. 2018;17:29.
Kiyotoki S, Nishikawa J, Sakaida I. Efficacy of Vonoprazan for Helicobacter pylori Eradication. Internal Medicine. 2020;59:153–161.
Murakami K, Sakurai Y, Shiino M, Funao N, Nishimura A, Asaka M. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut. 2016;65:1439–1446.
Graham DY, Optimal PPI. Dosing for Improving GERD Symptoms: Is Timing Everything? Dig. Dis Sci. 2019;64:4–6.
Mulford DJ, Leifke E, Hibberd M, Howden CW. The Effect of Food on the Pharmacokinetics of the Potassium-Competitive Acid Blocker Vonoprazan. Clin Pharmacol Drug Dev. 2022;11:278–284.
Scarpignato C, Leifke E, Smith N et al. A Population Pharmacokinetic Model of Vonoprazan: Evaluating the Effects of Race Disease Status, and Other Covariates on Exposure. J Clin Pharmacol. 2022;62:801–811.
Graham DY, Smith JL, Bouvet AA. What happens to tablets and capsules in the stomach: endoscopic comparison of disintegration and dispersion characteristics of two microencapsulated potassium formulations. J. Pharm. Sci. 1990;79:420–424.
HERTZ AF. THE MOTOR FUNCTIONS OF THE STOMACH QJM: An International Journal of Medicine. 1910;os3:373–394.
Kihira K, Satoh K, Saifuku K et al. Endoscopic topical therapy for the treatment of Helicobacter pylori infection. J. Gastroenterol. 1996;31:66–68.
Shiotani A, Roy P, Lu H, Graham DY. Helicobacter pylori diagnosis and therapy in the era of antimicrobial stewardship. Therap Adv Gastroenterol. 2021;14:17562848211064080.
Hopkins RJ. Current FDA-approved treatments for Helicobacter pylori and the FDA approval process. Gastroenterology. 1997;113:S126–S130.
Graham DY, Moss SF. Antimicrobial Susceptibility Testing for Helicobacter pylori Is Now Widely Available: When. How, Why Am J Gastroenterol. 2022;117:524–528.
Graham DY. Vonoprazan Helicobacter pylori eradication therapy: ethical and interpretation issues.Gut. 2017;66:384–386.
Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;6:800–808.
Li M, Oshima T, Horikawa Tet al. . Systematic review with meta-analysis: Vonoprazan, a potent acid blocker, is superior to proton-pump inhibitors for eradication of clarithromycin-resistant strains of Helicobacter pylori Helicobacter. 2018;23:e12495; Sue S, Ogushi M, Arima Iet al. . Vonoprazan- vs proton-pump inhibitor-based first-line 7-day triple therapy for clarithromycin-susceptible Helicobacter pylori: A multicenter, prospective, randomized trial Helicobacter. 2018;23:e12456.
Jenkins H, Sakurai Y, Nishimura Aet al. . Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects Aliment. Pharmacol. Ther. 2015;41:636–648; Sakurai Y, Nishimura A, Kennedy Get al. . Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (Vonoprazan) doses in healthy male Japanese/non-Japanese subjects Clin Transl. Gastroenterol. 2015;6:e94.
Gunaratne AW, Hamblin H, Clancy Aet al. . Combinations of antibiotics and vonoprazan for the treatment of Helicobacter pylori infections—Exploratory study Helicobacter. 2021;26:e12830.
Suzuki T, Kagami T, Uotani Tet al. . Comparison of effect of an increased dosage of vonoprazan versus vonoprazan plus lafutidine on gastric acid inhibition and serum gastrin European Journal of Clinical Pharmacology. 2018;74:45–52;
Sugimoto M, Furuta T, Shirai Net al. . Comparison of an increased dosage regimen of rabeprazole versus a concomitant dosage regimen of famotidine with rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotypes Clinical Pharmacology & Therapeutics. 2005;77:302–311.
Sugimoto M, Graham DY. High-dose versus standard-dose PPI in triple therapy for Helicobacter pylori eradication Nat. Clin. Pract. Gastroenterol. Hepatol. 2009;6:138–139.
Graham DY. Hp-normogram (normo-graham) for assessing the outcome of H. pylori therapy: Effect of resistance, duration, and CYP2C19 genotype Helicobacter. 2015;21:85–90.
Suzuki S, Gotoda T, Kusano C et al. Seven-day vonoprazan and low-dose amoxicillin dual therapy as first-line Helicobacter pylori treatment: a multicentre randomised trial in Japan Gut. 2020;69:1019–1026.
Graham DY, Lu H, Shiotani A. Vonoprazan-containing Helicobacter pylori triple therapies contribution to global antimicrobial resistance J Gastroenterol Hepatol. 2021;36:1159–1163.
Blaser MJ Missing microbes: how the overuse of antibiotics is fueling our modern plagues. New York: Macmillan; 2014;. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. W. H. O, City; World Health Organization; Tacconelli E, Pezzani MD. Public health burden of antimicrobial resistance in Europe The Lancet Infectious Diseases. 2017;2019:4–6.
Selection WECot, Medicines UoE, Organization WH The Selection and Use of Essential Medicines: Report of the WHO Expert Committee, 2013 (including the 18th WHO Model List of Essential Medicines and the 4th WHO Model List of Essential Medicines for Children). City: World Health Organization; 2014.
The 2019 WHO AWaRe classification of antibiotics for evaluation and monitoring of use. World Health Organization; 2019. (WHO/EMP/IAU/2019.11)
Dang BN, Graham DY. Helicobacter pylori infection and antibiotic resistance: a WHO high priority? Nat. Rev. Gastroenterol Hepatol. 2017;7:383–384
Ouyang Y, Wang M, Xu Y-L, Zhu Y, Lu N-H, Hu Y. Amoxicillin-vonoprazan dual therapy for Helicobacter pylori eradication: A systematic review and meta-analysis. Journal of Gastroenterology and Hepatology. 2022;37:1666–1672.
Funding
Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the final manuscript, and each meets the criteria for authorship established by the International Committee of Medical Journal Editors and verify the validity of the results reported.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Graham is a consultant for RedHill Biopharma and Phathom Pharmaceuticals regarding novel H. pylori therapies and has received research support for culture of Helicobacter pylori. He is also a consultant with Janssen Research & Development regarding potential gastrointestinal effects of drugs under development and has collaborated on research projects with American Molecular regarding molecular diagnostics for H. pylori.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Graham, D.Y. Why the Vonoprazan Helicobacter pylori Therapies in the US-European Trial Produced Unacceptable Cure Rates. Dig Dis Sci 68, 1691–1697 (2023). https://doi.org/10.1007/s10620-023-07886-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-07886-5