Abstract
Background
People with inflammatory bowel disease (IBD) including ulcerative colitis are at risk for colorectal cancer. Despite available effective drugs used to treat IBD, many patients fail or lose response over time with some displaying drug-induced adverse events. Saffron (Crocus sativus) has been reported to have anti-inflammatory properties. Its protective role in IBD has not been explored extensively.
Aim
To establish whether saffron treatment alleviates inflammation in experimental colitis.
Methods
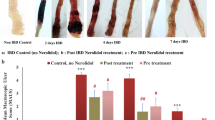
Colitis was induced in C57BL/6 mice with 3% DSS and treated with either saffron doses (7.5, 15, 20, 25 mg/kg body weight) or vehicle through daily gavage. On day 11, mice were euthanized and analyzed for gross and microscopic inflammation. Distal colon segments were collected for mRNA and protein expression of HO-1 protein and GPX2, (the downstream targets of NRF-2). Nrf-2 translocation from cytosol to nucleus was confirmed by immunofluorescence, and further Nrf-2 protein expression in nuclear and cytosolic fraction of colon was analyzed by immunoblot. Immune cells were isolated from the lamina propria of mouse colon for flow cytometry-based immunophenotyping. Colitis was also induced in C57BL/6 Ahr knockout and wild type mice to explore the involvement of Ahr-dependent pathways in saffron’s protective effect(s). The therapeutic effect of saffron was further validated in another TNBS model of colitis.
Results
Saffron 20 mg/kg body weight showed improved colon gross and histology features and led to better body weight, colon length, histology score, and reduced disease activity index (DAI). Saffron significantly decreased pro-inflammatory macrophages (M1), while increasing anti-inflammatory macrophages (M2) and IL10 + dendritic cells. Saffron treatment also enhanced CD3 + T and CD3 + CD8 + T cells followed by increase in different CD3 + CD4 + T cells subsets like CD25 + T cells, FoxP3 + CD25 + regulatory T cells, and CD4 + FOXP3 + CD25-regulatory T cells. Immunoblot analysis showed a significant increase in HO-1/GPX2 protein expression. With saffron treatment, Nrf-2 translocation into nucleus from cytosol also supports the involvement of Nrf-2 and its downstream targets in the protective effect of saffron. Further, we demonstrated that saffron in part exert anti-inflammatory effect through activation of aryl hydrocarbon receptor (AhR)-nuclear factor erythroid 2–related factor 2 (Nrf2)-dependent pathways.
Conclusion
These data demonstrate saffron’s therapeutic potential and its protective role in part via Ahr/Nrf-2 pathways and regulatory innate and adaptive immune cells.







Similar content being viewed by others
Abbreviations
- IBD:
-
Inflammatory bowel disease
- DSS:
-
Dextran sodium sulfate
- NRF-2:
-
Nuclear factor erythroid 2
- Ahr:
-
Aryl hydrocarbon receptor
- HO-1:
-
Hemeoxygenase-1
- GPX2:
-
Glutathione peroxidase 2
- TNF-α:
-
Tumor necrosis factor-alpha
- DAI:
-
Disease activity index
- DCs:
-
Dendritic cells
- CD:
-
Crohn’s disease
- UC:
-
Ulcerative colitis
- SFE:
-
Saffron aqueous extract
- TNBS:
-
Trinitrobenzenesulfonic acid
- HBSS:
-
Hank's balanced salt solution (HBSS)
- BCS:
-
Bovine calf serum
References
Molodecky NA et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142:46-54.e42 (quiz e30).
Malhotra A et al. All-cause hospitalizations for inflammatory bowel diseases: Can the reason for admission provide information on inpatient resource use? A study from a large veteran affairs hospital. Mil Med Res 2016;3:28.
Kelsen J, Baldassano RN. Inflammatory bowel disease: the difference between children and adults. Inflamm Bowel Dis 2008;14:S9-11.
Travis S. Is IBD different in the elderly? Inflamm Bowel Dis 2008;14:S12–S13.
Charpentier C et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut 2014;63:423–432.
Beaugerie L et al. Risk of new or recurrent cancer under immunosuppressive therapy in patients with IBD and previous cancer. Gut 2014;63:1416–1423.
Jostins L et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119–124.
de Lange KM et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet 2017;49:256–261.
Cho EJ et al. Anti-inflammatory effects of methanol extract of Patrinia scabiosaefolia in mice with ulcerative colitis. J Ethnopharmacol 2011;136:428–435.
Sergent T et al. Anti-inflammatory effects of dietary phenolic compounds in an in vitro model of inflamed human intestinal epithelium. Chem Biol Interact 2010;188:659–667.
Celiberto LS et al. Inflammatory bowel disease and immunonutrition: novel therapeutic approaches through modulation of diet and the gut microbiome. Immunology 2018;155:36–52.
Triantafillidis JK et al. Favorable results from the use of herbal and plant products in inflammatory bowel disease: evidence from experimental animal studies. Ann Gastroenterol 2016;29:268–281.
Debnath T, Kim DH, Lim BO. Natural products as a source of anti-inflammatory agents associated with inflammatory bowel disease. Molecules 2013;18:7253–7270.
Langmead L, Rampton DS. Review article: complementary and alternative therapies for inflammatory bowel disease. Aliment Pharmacol Ther 2006;23:341–349.
Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev 2004;28:426–432.
Siddiqui MJ et al. Saffron (Crocus sativus L.): As an antidepressant. J Pharm Bioallied Sci 2018;10:173–180.
Festuccia C et al. Crocetin and crocin from saffron in cancer chemotherapy and chemoprevention. Anticancer Agents Med Chem 2018;19:38.
Ashktorab H et al. Saffron: the golden spice with therapeutic properties on digestive diseases. Nutrients 2019;11:943.
Pitsikas N, Tarantilis PA. Effects of the active constituents of Crocus sativus L. crocins and their combination with memantine on recognition memory in rats. Behav Pharmacol 2018;29:400–412.
Khorasany AR, Hosseinzadeh H. Therapeutic effects of saffron (Crocus sativus L.) in digestive disorders: a review. Iran J Basic Med Sci 2016;19:455–469.
Bakshi H et al. DNA fragmentation and cell cycle arrest: a hallmark of apoptosis induced by crocin from Kashmiri saffron in a human pancreatic cancer cell line. Asian Pac J Cancer Prev 2010;11:675–679.
Aung HH et al. Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp Oncol 2007;29:175–180.
Noureini SK, Wink M. Antiproliferative effects of crocin in HepG2 cells by telomerase inhibition and hTERT down-regulation. Asian Pac J Cancer Prev 2012;13:2305–2309.
Tabtabaei S et al. Geographical classification of Iranian and Italian saffron sources based on HPLC analysis and UV–Vis spectra of aqueous extracts. Eur Food Res Technol 2019;245:2435–2446.
Erben U et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol 2014;7:4557–4576.
Wirtz S et al. Chemically induced mouse models of intestinal inflammation. Nat Protoc 2007;2:541–546.
Koboziev I et al. Pharmacological intervention studies using mouse models of the inflammatory bowel diseases: translating preclinical data into new drug therapies. Inflamm Bowel Dis 2011;17:1229–1245.
Pandurangan AK et al. Gallic acid attenuates dextran sulfate sodium-induced experimental colitis in BALB/c mice. Drug Des Dev Ther 2015;9:3923–3934.
Chassaing B et al. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS ONE 2012;7:e44328.
Nguyen LP et al. Role and species-specific expression of colon T cell homing receptor GPR15 in colitis. Nat Immunol 2015;16:207–213.
Kim J, Zarjou A, Traylor AM, Bolisetty S, Jaimes EA, Hull TD, George JF, Mikhail FM, Agarwal A. In vivo regulation of the heme oxygenase-1 gene in humanized transgenic mice. Kidney Int 2012;82(3):278–291. https://doi.org/10.1038/ki.2012.102.
Aoki R, Aoki-Yoshida A, Suzuki C, Takayama Y, Slominski AT. Protective effect of indole-3-pyruvate against ultraviolet b-induced damage to cultured HaCaT keratinocytes and the skin of hairless mice. PloS one. 2014;9(5):e96804. https://doi.org/10.1371/journal.pone.0096804.
Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucl Acids Res 2001;29:e45.
Rubin SJS et al. Mass cytometry reveals systemic and local immune signatures that distinguish inflammatory bowel diseases. Nat Commun 2019;10:2686.
Ashktorab H et al. Inflammatory polyps occur more frequently in inflammatory bowel disease than other colitis patients. BMC Gastroenterol 2020;20:170.
Kiesler P, Fuss IJ, Strober W. Experimental models of inflammatory bowel diseases. Cell Mol Gastroenterol Hepatol 2015;1:154–170.
Luissint AC, Parkos CA, Nusrat A. Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology 2016;151:616–632.
Neurath M. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol 2017;14:688.
Kawabata K et al. Dietary crocin inhibits colitis and colitis-associated colorectal carcinogenesis in male ICR mice. Evid Based Complement Altern Med 2012;2012:820415.
Cote-Daigneault J et al. Biologics in inflammatory bowel disease: what are the data? United Eur Gastroenterol J 2015;3:419–428.
Bostan HB, Mehri S, Hosseinzadeh H. Toxicology effects of saffron and its constituents: a review. Iran J Basic Med Sci 2017;20:110–121.
Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology 2018;154:3–20.
Rutella S, Locatelli F. Intestinal dendritic cells in the pathogenesis of inflammatory bowel disease. World J Gastroenterol 2011;17:3761–3775.
Khodir AE et al. Targeting Nrf2/HO-1 signaling by crocin: Role in attenuation of AA-induced ulcerative colitis in rats. Biomed Pharmacother 2019;110:389–399.
Lertnimitphun P et al. Safranal alleviates dextran sulfate sodium-induced colitis and suppresses macrophage-mediated inflammation. Front Pharmacol 2019;10:1281.
Mitsuishi Y, Motohashi H, Yamamoto M. The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism. Front Oncol 2012;2:200.
Singh R et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun 2019;10:89.
Ishizuka F et al. Crocetin, a carotenoid derivative, inhibits retinal ischemic damage in mice. Eur J Pharmacol 2013;703:1–10.
Yamauchi M et al. Crocetin prevents retinal degeneration induced by oxidative and endoplasmic reticulum stresses via inhibition of caspase activity. Eur J Pharmacol 2011;650:110–119.
Acknowledgments
We would like to thank Gulf Pearls SPRL (Brussels, Belgium, www.gp-food.com) that provided saffron for our research studies. In addition, we would like to thank all the saffron growers and people involved in the production of this golden spice.
Funding
This project was supported (in part) by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number G12MD007597.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, G., Haileselassie, Y., Ji, A.R. et al. Protective Effect of Saffron in Mouse Colitis Models Through Immune Modulation. Dig Dis Sci 67, 2922–2935 (2022). https://doi.org/10.1007/s10620-021-07163-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07163-3