Abstract
Background
DEAD-Box Helicase 3 X-Linked (DDX3X) is a member of the DEAD-box helicases that play a crucial role in RNA metabolism. Although DDX3X has been shown to contribute to tumorigenesis, the detailed mechanisms by which DDX3X functions in pancreatic ductal adenocarcinoma (PDAC) biogenesis remain poorly understood.
Aims
The goal of the present study was to elucidate the molecular mechanisms by which DDX3X contributes to tumorigenesis in PDAC.
Methods
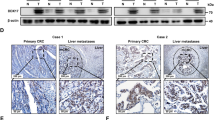
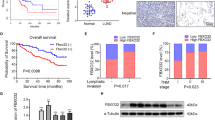
Kaplan–Meier curves, the log-rank test, t test and Cox regression were used to analyze the relationship between DDX3X expression and the clinicopathological features of PDAC patients. DDX3X and p62 expression in human PDAC tissues was analyzed by immunohistochemistry. Monolayer scratch healing assays, cell migration assays and nude mouse lung metastasis models were used to evaluate the effect of DDX3X on metastasis in vitro and in vivo. Western blot analysis was used to assess the expression of proteins in the signaling pathway.
Results
We authenticated high DDX3X expression was associated with a poor prognosis in PDAC. The loss of DDX3X attenuated the migratory capacity of PDAC cells in vitro and in vivo. DDX3X was shown to facilitate epithelial-mesenchymal transition (EMT) and the phosphorylation of p65 and eIF2α. Moreover, DDX3X displayed oncogenic activity by promoting p62 accumulation.
Conclusions
Our results demonstrated that DDX3X activates NF-κB and promotes metastasis by inducing EMT via p62.





Similar content being viewed by others
References
Soto-Rifo R, Ohlmann T. The role of the DEAD-box RNA helicase DDX3 in mRNA metabolism. Wiley Interdiscip Rev RNA. 2013;4:369–385.
Kerr CL, Bol GM, Vesuna F, et al. Targeting RNA helicase DDX3 in stem cell maintenance and teratoma formation. Genes Cancer. 2019;10:11–20.
Samir P, Kesavardhana S, Patmore DM, et al. DDX3X acts as a live-or-die checkpoint in stressed cells by regulating NLRP3 inflammasome. Nature. 2019;573:590–594.
Chao CH, Chen CM, Cheng PL, et al. DDX3, a DEAD box RNA helicase with tumor growth-suppressive property and transcriptional regulation activity of the p21waf1/cip1 promoter, is a candidate tumor suppressor. Cancer Res. 2006;66:6579–6588.
Vellky JE, Ricke EA, Huang W, et al. Expression and localization of DDX3 in prostate cancer progression and metastasis. Am J Pathol. 2019;189:1256–1267.
Botlagunta M, Vesuna F, Mironchik Y, et al. Oncogenic role of DDX3 in breast cancer biogenesis. Oncogene. 2008;27:3912–3922.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
Ling J, Kang Y, Zhao R, et al. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105–120.
Yang H, Cui J, Shi J, et al. Endoplasmic reticulum stress participates in inflammation-accelerated, lipid-mediated injury of human glomerular mesangial cells. Nephrology (Carlton, Vic). 2017;22:234–242.
Paul S, Traver MK, Kashyap AK, et al. T cell receptor signals to NF-kappaB are transmitted by a cytosolic p62-Bcl10-Malt1-IKK signalosome. Sci Signal. 2014;7:45.
Zotti T, Scudiero I, Settembre P, et al. TRAF6-mediated ubiquitination of NEMO requires p62/sequestosome-1. Mol Immunol. 2014;58:27–31.
Cubillos-Ruiz JR, Bettigole SE, Glimcher LH. Tumorigenic and immunosuppressive effects of endoplasmic reticulum stress in cancer. Cell. 2017;168:692–706.
Rodriguez-Aznar E, Wiesmüller L, Sainz B Jr, et al. EMT and stemness-key players in pancreatic cancer stem cells. Cancers. 2019;11:1136.
Ayres Pereira M, Chio IIC. Metastasis in pancreatic ductal adenocarcinoma: current standing and methodologies. Genes. 2019;11:6.
Umemura A, He F, Taniguchi K, et al. p62, Upregulated during preneoplasia, induces hepatocellular carcinogenesis by maintaining survival of stressed HCC-initiating cells. Cancer Cell. 2016;29:935–948.
Iwadate R, Inoue J, Tsuda H, et al. High expression of p62 protein is associated with poor prognosis and aggressive phenotypes in endometrial cancer. Am J Pathol. 2015;185:2523–2533.
Inami Y, Waguri S, Sakamoto A, et al. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol. 2011;193:275–284.
Mathew R, Karp CM, Beaudoin B, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075.
Duran A, Linares JF, Galvez AS, et al. The signaling adaptor p62 is an important NF-kappaB mediator in tumorigenesis. Cancer Cell. 2008;13:343–354.
Guttridge DC, Albanese C, Reuther JY, et al. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799.
Kang YK, Putluri N, Maity S, et al. CAPER is vital for energy and redox homeostasis by integrating glucose-induced mitochondrial functions via ERR-α-Gabpa and stress-induced adaptive responses via NF-κB-cMYC. PLoS Genet. 2015;11:e1005116.
Acknowledgments
All authors have read and approved the final manuscript. This research was supported by the Grants from Shaanxi Provincial Central Committee’s Special Fund Plan for Guiding Local Science and Technology Development, No. 2016ZY-HM-01.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, Y., Xu, B., Zhao, Y. et al. DEAD-Box Helicase 3 X-Linked Promotes Metastasis by Inducing Epithelial-Mesenchymal Transition via p62/Sequestosome-1. Dig Dis Sci 66, 3893–3902 (2021). https://doi.org/10.1007/s10620-020-06735-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06735-z