Abstract
Background
The renin–angiotensin system (RAS) is activated in inflammatory bowel disease (IBD), and vitamin D deficiency aggravates the development of colitis, but the relationship between the local colonic RAS and vitamin D is unclear with regard to the pathogenesis of IBD.
Aims
To investigate whether vitamin D suppresses the local colonic RAS to prevent colonic mucosal inflammation in a mouse model of experimental colitis.
Methods
C57BL/6 mice fed vitamin D-deficient (VDD) diet for 8 weeks were induced to colitis by 2,4,6-trinitrobenzenesulfonic acid (TNBS), with mice fed vitamin D-sufficient (VDS) diet as controls. Colitis severity was assessed by histology, and pro-inflammatory cytokines, RAS components, and signaling pathways were quantified by real-time RT-PCR and Western blotting.
Results
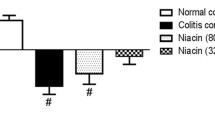
C57BL/6 mice fed the VDD diet for 8 weeks exhibited significantly lower serum 25(OH)D3 concentrations compared to mice fed the VDS diet. When these VDD mice were induced to colitis by TNBS, they exhibited more severe colonic inflammation and developed more severe colitis compared to the VDS counterparts. VDD diet feeding resulted in higher production of mucosal pro-inflammatory cytokines, higher activation of the myosin light chain kinase-tight junction regulatory pathway, and greater increases in mucosal permeability. VDD diet feeding also enhanced colonic RAS activation. Treatment with angiotensin II receptor blocker losartan markedly alleviated colitis in TNBS-induced VDD mice.
Conclusion
Vitamin D deficiency promotes colonic inflammation at least in part due to over activation of the local RAS in the colon.




Similar content being viewed by others
References
Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429.
Watson AJ, Chu S, Sieck L, et al. Epithelial barrier function in vivo is sustained despite gaps in epithelial layers. Gastroenterology. 2005;129:902–912.
Fasano A, Shea-Donohue T. Mechanisms of disease: The role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2:416–422.
Clayburgh DR, Barrett TA, Tang Y, et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715.
Shen L, Black ED, Witkowski ED, et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci. 2006;119:2095–2106.
Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694.
Qiu W, Wu B, Wang X, et al. PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. J Clin Invest. 2011;121:1722–1732.
Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin–angiotensin systems. Physiol Rev. 2006;86:747–803.
Fandriks L. The renin–angiotensin system and the gastrointestinal mucosa. Acta Physiol (Oxf). 2011;201:157–167. https://doi.org/10.1111/j.1748-1716.2010.02165.x.
Geara AS, Azzi J, Jurewicz M, Abdi R. The renin–angiotensin system: An old, newly discovered player in immunoregulation. Transpl Rev (Orlando). 2009;23:151–158. https://doi.org/10.1016/j.trre.2009.04.002.
Jaszewski R, Tolia V, Ehrinpreis MN, et al. Increased colonic mucosal angiotensin I and II concentrations in Crohn’s colitis. Gastroenterology. 1990;98:1543–1548.
He L, Du J, Chen Y, et al. renin–angiotensin system promotes colonic inflammation by inducing TH17 activation via JAK2/STAT pathway. Am J Physiol Gastrointest Liver Physiol. 2019;316:G774–G784. https://doi.org/10.1152/ajpgi.00053.2019.
Mizushima T, Sasaki M, Ando T, et al. Blockage of angiotensin II type 1 receptor regulates TNF-alpha-induced MAdCAM-1 expression via inhibition of NF-kappaB translocation to the nucleus and ameliorates colitis. Am J Physiol Gastrointest Liver Physiol. 2010;298:G255–G266. https://doi.org/10.1152/ajpgi.00264.2009.
Wengrower D, Zanninelli G, Pappo O, et al. Prevention of fibrosis in experimental colitis by captopril: The role of tgf-beta1. Inflamm Bowel Dis. 2004;10:536–545.
Spencer AU, Yang H, Haxhija EQ, Wildhaber BE, Greenson JK, Teitelbaum DH. Reduced severity of a mouse colitis model with angiotensin converting enzyme inhibition. Dig Dis Sci. 2007;52:1060–1070. https://doi.org/10.1007/s10620-006-9124-2.
Inokuchi Y, Morohashi T, Kawana I, Nagashima Y, Kihara M, Umemura S. Amelioration of 2,4,6-trinitrobenzene sulphonic acid induced colitis in angiotensinogen gene knockout mice. Gut. 2005;54:349–356.
Katada K, Yoshida N, Suzuki T, et al. Dextran sulfate sodium-induced acute colonic inflammation in angiotensin II type 1a receptor deficient mice. Inflamm Res. 2008;57:84–91. https://doi.org/10.1007/s00011-007-7098-y.
Shi Y, Liu T, He L, et al. Activation of the renin–angiotensin system promotes colitis development. Sci Rep. 2016;6:27552. https://doi.org/10.1038/srep27552.
Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–776.
Del Pinto R, Pietropaoli D, Chandar AK, Ferri C, Cominelli F. Association between inflammatory bowel disease and vitamin D deficiency: A systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:2708–2717. https://doi.org/10.1097/MIB.0000000000000546.
Ham M, Longhi MS, Lahiff C, Cheifetz A, Robson S, Moss AC. Vitamin D levels in adults with Crohn’s disease are responsive to disease activity and treatment. Inflamm Bowel Dis. 2014;20:856–860. https://doi.org/10.1097/MIB.0000000000000016.
Ulitsky A, Ananthakrishnan AN, Naik A, et al. Vitamin D deficiency in patients with inflammatory bowel disease: Association with disease activity and quality of life. JPEN. 2011;35:308–316.
Meckel K, Li YC, Lim J, et al. Serum 25-hydroxyvitamin D concentration is inversely associated with mucosal inflammation in patients with ulcerative colitis. Am J Clin Nutr. 2016;104:113–120. https://doi.org/10.3945/ajcn.115.123786.
Ananthakrishnan AN, Khalili H, Higuchi LM, et al. Higher predicted vitamin D status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012;142:482–489. https://doi.org/10.1053/j.gastro.2011.11.040.
Du J, Chen Y, Shi Y, et al. 1,25-Dihydroxyvitamin D protects intestinal epithelial barrier by regulating the myosin light chain kinase signaling pathway. Inflamm Bowel Dis. 2015;21:2495–2506. https://doi.org/10.1097/MIB.0000000000000526.
Du J, Wei X, Ge X, Chen Y, Li YC. Microbiota-dependent induction of colonic Cyp27b1 is associated with colonic inflammation: Implications of locally produced 1,25-dihydroxyvitamin D3 in inflammatory regulation in the colon. Endocrinology. 2017;158:4064–4075. https://doi.org/10.1210/en.2017-00578.
He L, Liu T, Shi Y, et al. Gut epithelial vitamin D receptor regulates microbiota-dependent mucosal inflammation by suppressing intestinal epithelial cell apoptosis. Endocrinology. 2018;159:967–979. https://doi.org/10.1210/en.2017-00748.
Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin–angiotensin system. J Clin Invest. 2002;110:229–238.
Li YC. Vitamin D regulation of the renin–angiotensin system. J Cell Biochem. 2003;88:327–331.
Qiao G, Kong J, Uskokovic M, Li YC. Analogs of 1alpha,25-dihydroxyvitamin D3 as novel inhibitors of renin biosynthesis. J Steroid Biochem Mol Biol. 2005;96:59–66.
Kong J, Qiao G, Zhang Z, Liu SQ, Li YC. Targeted vitamin D receptor expression in juxtaglomerular cells suppresses renin expression independent of parathyroid hormone and calcium. Kidney Int. 2008;74:1577–1581.
Yuan W, Pan W, Kong J, et al. 1,25-Dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic amp response element in the renin gene promoter. J Biol Chem. 2007;282:29821–29830.
Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116:2062–2072.
Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: A negative endocrine regulator of the renin–angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89–90:387–392.
Dougherty U, Mustafi R, Sadiq F, et al. The renin–angiotensin system mediates EGF receptor-vitamin d receptor cross-talk in colitis-associated colon cancer. Clin Cancer Res. 2014;20:5848–5859. https://doi.org/10.1158/1078-0432.CCR-14-0209.
Liu W, Chen Y, Golan MA, et al. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest. 2013;123:3983–3996. https://doi.org/10.1172/JCI65842.
Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546.
Li YC, Bolt MJG, Cao L-P, Sitrin MD. Effects of vitamin D receptor inactivation on the expression of calbindins and calcium metabolism. Am J Physiol Endocrinol Metab. 2001;281:E558–E564.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108.
Lim WC, Hanauer SB, Li YC. Mechanisms of disease: Vitamin D and inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:308–315.
Driscoll RH Jr, Meredith SC, Sitrin M, Rosenberg IH. Vitamin D deficiency and bone disease in patients with Crohn’s disease. Gastroenterology. 1982;83:1252–1258.
Harries AD, Brown R, Heatley RV, Williams LA, Woodhead S, Rhodes J. Vitamin D status in Crohn’s disease: Association with nutrition and disease activity. Gut. 1985;26:1197–1203.
Pappa HM, Gordon CM, Saslowsky TM, et al. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics. 2006;118:1950–1961.
Raftery T, Martineau AR, Greiller CL, et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: Results from a randomised double-blind placebo-controlled study. United Eur Gastroenterol J. 2015;3:294–302. https://doi.org/10.1177/2050640615572176.
Li J, Chen N, Wang D, Zhang J, Gong X. Efficacy of vitamin D in treatment of inflammatory bowel disease: A meta-analysis. Medicine (Baltimore). 2018;97:e12662. https://doi.org/10.1097/MD.0000000000012662.
Salmenkari H, Holappa M, Forsgard RA, Korpela R, Vapaatalo H. Orally administered angiotensin-converting enzyme-inhibitors captopril and isoleucine–proline–proline have distinct effects on local renin–angiotensin system and corticosterone synthesis in dextran sulfate sodium-induced colitis in mice. J Physiol Pharmacol. 2017;68:355–362.
Okawada M, Wilson MW, Larsen SD, Lipka E, Hillfinger J, Teitelbaum DH. Blockade of the renin–angiotensin system prevents acute and immunologically relevant colitis in murine models. Pediatr Surg Int. 2016;32:1103–1114. https://doi.org/10.1007/s00383-016-3965-3.
Jacobs JD, Wagner T, Gulotta G, et al. Impact of angiotensin II signaling blockade on clinical outcomes in patients with inflammatory bowel disease. Dig Dis Sci. 2019. https://doi.org/10.1007/s10620-019-5474-4.
Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: Blockade of compensatory renin increase. Proc Natl Acad Sci USA. 2008;105:15896–15901.
Deb DK, Sun T, Wong KE, et al. Combined vitamin D analog and AT1 receptor antagonist synergistically block the development of kidney disease in a model of type 2 diabetes. Kidney Int. 2010;77:1000–1009.
de Borst MH, Hajhosseiny R, Tamez H, Wenger J, Thadhani R, Goldsmith DJ. Active vitamin D treatment for reduction of residual proteinuria: A systematic review. J Am Soc Nephrol. 2013;24:1863–1871. https://doi.org/10.1681/ASN.2013030203.
de Zeeuw D, Agarwal R, Amdahl M, et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): A randomised controlled trial. Lancet. 2010;376:1543–1551.
Acknowledgments
This work was supported in part by a research Grant from Liaoning Province.
Author information
Authors and Affiliations
Contributions
YCL conceived and designed the research; XW, XL, XG, and YS performed the research; JD, XL, ZX, WL, and Z-YW provided technical assistance; XW and YCL performed the data analyses; XW and YCL wrote the manuscript; and Z-YW and YCL supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
All authors have declared that no conflict of interest exists in this work.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, X., Li, X., Du, J. et al. Vitamin D Deficiency Exacerbates Colonic Inflammation Due to Activation of the Local Renin–Angiotensin System in the Colon. Dig Dis Sci 66, 3813–3821 (2021). https://doi.org/10.1007/s10620-020-06713-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06713-5