Abstract
Background
Hyperbaric oxygen therapy (HBOT) improves short-term outcomes for ulcerative colitis (UC) patients hospitalized for acute flares. Longer-term impacts and cost-effectiveness are unknown.
Methods
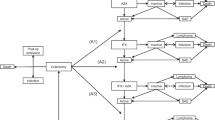
We compared disease outcomes and cost-effectiveness of HBOT in addition to standard of care versus standard of care alone for UC patients hospitalized for acute flares using a microsimulation model. Published literature was used for transition probabilities, costs, and quality-adjusted life year (QALY) estimates. We modeled 100,000 individuals in each group over a 5-year horizon and compared rates of re-hospitalization, rescue medical therapy, colectomy, death, and cost-effectiveness at a willingness-to-pay of $100,000/QALY. Probabilistic sensitivity analyses were performed with 500 samples and 250 trials, in addition to multiple microsimulation sensitivity analyses.
Results
The use of HBOT at the time of index hospitalization for an acute UC flare is projected to reduce the risk of re-hospitalization, inpatient rescue medical therapy, and inpatient emergent colectomy by over 60% (p < 0.001) and mortality by over 30% (p <0.001), during a 5-year horizon. The HBOT strategy costs more ($5600 incremental cost) but also yielded higher QALYs (0.13 incremental yield), resulting in this strategy being cost-effective ($43,000/QALY). Results were sensitive to HBOT costs and rates of endoscopic improvement with HBOT. Probabilistic sensitivity analyses observed HBOT to be more cost-effective than standard of care in 95% of iterations.
Conclusion
The use of HBOT to optimize response to steroids during the index hospitalization for an acute UC flare is cost-effective and is projected to result in significant reductions in disease-related complications in the long term.




Similar content being viewed by others
References
Fumery M, Singh S, Dulai PS, Gower-Rousseau C, Peyrin-Biroulet L, Sandborn WJ. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2018;16:343–356 e343.
Ma C, Smith M, Guizzetti L, et al. Assessing national trends and disparities in ambulatory, emergency department, and inpatient visits for inflammatory bowel disease in the United States (2005–2016). Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc.. 2020;8:2500–2509.e1.
Thomas MG, Bayliss C, Bond S, et al. Trial summary and protocol for a phase II randomised placebo-controlled double-blinded trial of Interleukin 1 blockade in Acute Severe Colitis: the IASO trial. BMJ Open. 2019;9:e023765.
Williams JG, Alam MF, Alrubaiy L, et al. Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (CONSTRUCT): a mixed methods, open-label, pragmatic randomised trial. Lancet Gastroenterol Hepatol. 2016;1:15–24.
Dulai PS, Jairath V. Acute severe ulcerative colitis: latest evidence and therapeutic implications. Ther Adv Chronic Dis. 2018;9:65–72.
Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2007;5:103–110.
Singh S, Al-Darmaki A, Frolkis AD, et al. Postoperative mortality among patients with inflammatory bowel diseases: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2015;149:928–937.
de Silva S, Ma C, Proulx MC, et al. Postoperative complications and mortality following colectomy for ulcerative colitis. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2011;9:972–980.
Narula N, Marshall JK, Colombel JF, et al. Systematic review and meta-analysis: infliximab or cyclosporine as rescue therapy in patients with severe ulcerative colitis refractory to steroids. Am J Gastroenterol. 2016;111:477–491.
Vedamurthy A, Xu L, Luther J, et al. Long-term outcomes of immunosuppression-naïve steroid responders following hospitalization for ulcerative colitis. Dig Dis Sci. 2018;63:2740–2746. https://doi.org/10.1007/s10620-018-5176-3.
Dulai PS, Gleeson MW, Taylor D, Holubar SD, Buckey JC, Siegel CA. Systematic review: the safety and efficacy of hyperbaric oxygen therapy for inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:1266–1275.
Dulai PS, Buckey JC Jr, Raffals LE, et al. Hyperbaric oxygen therapy is well tolerated and effective for ulcerative colitis patients hospitalized for moderate-severe flares: a phase 2A pilot multi-center, randomized, double-blind, sham-controlled trial. Am J Gastroenterol. 2018;113:1516–1523.
Dulai PS. A phase 2B randomized trial of hyperbaric oxygen therapy for ulcerative colitis patients hospitalized for moderate to severe flares. Aliment Pharmacol Ther.. 2020;52:955–963.
Borren NZ, Khalili H, Luther J, Colizzo FP, Garber JJ, Ananthakrishnan AN. Second-look endoscopy in hospitalized severe ulcerative colitis: a retrospective cohort study. Inflamm Bowel Dis. 2019;25:750–755.
Lee HS, Yang SK, Soh JS, et al. Short- and long-term outcomes of acute severe ulcerative colitis in Korea: the 1999–2005 cohort. Inflamm Bowel Dis. 2015;21:1825–1831.
Jain S, Kedia S, Sethi T, et al. Predictors of long-term outcomes in patients with acute severe colitis: a northern Indian cohort study. J Gastroenterol Hepatol. 2018;33:615–622.
Molnár T, Farkas K, Nyári T, Szepes Z, Nagy F, Wittmann T. Response to first intravenous steroid therapy determines the subsequent risk of colectomy in ulcerative colitis patients. J Gastrointestin Liver Dis. 2011;20:359–363.
Feuerstein JD, Isaacs KL, Schneider Y, Siddique SM, Falck-Ytter Y, Singh S. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020;158:1450–1461.
Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384–413.
Bitton A, Buie D, Enns R, et al. Treatment of hospitalized adult patients with severe ulcerative colitis: Toronto consensus statements. Am J Gastroenterol.. 2012;107:179–194. (author reply 195).
Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut. 1996;38:905–910.
Dulai PS, Sandborn WJ, Murphy J. Microsimulation model to determine the cost effectiveness of treat to target strategies for ulcerative colitis. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2020.
Mao EJ, Hazlewood GS, Kaplan GG, Peyrin-Biroulet L, Ananthakrishnan AN. Systematic review with meta-analysis: comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 2017;45:3–13.
Perera S YS, Stott-Miller M, Brady J. Analysis of healthcare resource utilization and costs after the initiation of biologic treatment in patients with ulcerative colitis and Crohn’s disease. JHEOR.
Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012;380:1909–1915.
Peyrin-Biroulet L, Germain A, Patel AS, Lindsay JO. Systematic review: outcomes and post-operative complications following colectomy for ulcerative colitis. Aliment Pharmacol Ther. 2016;44:807–816.
Lindsay JO, Bergman A, Patel AS, Alesso SM, Peyrin-Biroulet L. Systematic review: the financial burden of surgical complications in patients with ulcerative colitis. Aliment Pharmacol Ther. 2015;41:1066–1078.
Archer R, Tappenden P, Ren S, et al. Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy (including a review of TA140 and TA262): clinical effectiveness systematic review and economic model. Health Technol Assess (Winch, Engl). 2016;20:1–326.
Stawowczyk E, Kawalec P. A systematic review of the cost-effectiveness of biologics for ulcerative colitis. PharmacoEconomics. 2018;36:419–434.
Coward S, Heitman SJ, Clement F, et al. Ulcerative colitis-associated hospitalization costs: a population-based study. Can J Gastroenterol Hepatol. 2015;29:357–362.
Yokomizo L, Limketkai B, Park KT. Cost-effectiveness of adalimumab, infliximab or vedolizumab as first-line biological therapy in moderate-to-severe ulcerative colitis. BMJ Open Gastroenterol. 2016;3:e000093.
Wilson M, Lucas A, Cameron A, Luo M. budget impact of adding vedolizumab to a health plan formulary as another first-line biologic option for ulcerative colitis and crohn’s disease. Am Health Drug Benefits. 2018;11:253–262.
Milev S, DiBonaventura MD, Quon P, et al. An economic evaluation of tofacitinib for the treatment of moderately-to-severely active ulcerative colitis: modeling the cost of treatment strategies in the United States. J Med Econ. 2019;22:859–868.
Chaudhary MA, Fan T. Cost-effectiveness of infliximab for the treatment of acute exacerbations of ulcerative colitis in the Netherlands. Biol Ther. 2013;3:45–60.
Singh S, Murad MH, Fumery M, Dulai PS, Sandborn WJ. First- and second-line pharmacotherapies for patients with moderate to severely active ulcerative colitis: an updated network meta-analysis. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2020;18:2179–2191.e6.
Maser EA, Deconda D, Lichtiger S, Ullman T, Present DH, Kornbluth A. Cyclosporine and infliximab as rescue therapy for each other in patients with steroid-refractory ulcerative colitis. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2008;6:1112–1116.
Narula N, Fine M, Colombel JF, Marshall JK, Reinisch W. Systematic review: sequential rescue therapy in severe ulcerative colitis: do the benefits outweigh the risks? Inflamm Bowel Dis. 2015;21:1683–1694.
Nalagatla N, Falloon K, Tran G, et al. Effect of accelerated infliximab induction on short- and long-term outcomes of acute severe ulcerative colitis: a retrospective multicenter study and meta-analysis. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2019;17:502–509.e501.
Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–1103.
Laharie D, Bourreille A, Branche J, et al. Long-term outcome of patients with steroid-refractory acute severe UC treated with ciclosporin or infliximab. Gut. 2018;67:237–243.
Chen JH, Andrews JM, Kariyawasam V, et al. Review article: acute severe ulcerative colitis—evidence-based consensus statements. Aliment Pharmacol Ther. 2016;44:127–144.
Ordás I, Domènech E, Mañosa M, et al. Long-term efficacy and safety of cyclosporine in a cohort of steroid-refractory acute severe ulcerative colitis patients from the ENEIDA Registry (1989–2013): a Nationwide Multicenter Study. Am J Gastroenterol. 2017;112:1709–1718.
Mocciaro F, Renna S, Orlando A, et al. Cyclosporine or infliximab as rescue therapy in severe refractory ulcerative colitis: early and long-term data from a retrospective observational study. J Crohn’s Colitis. 2012;6:681–686.
Thorne K, Alrubaiy L, Akbari A, Samuel DG, Morrison-Rees S, Roberts SE. Colectomy rates in patients with ulcerative colitis following treatment with infliximab or ciclosporin: a systematic literature review. Eur J Gastroenterol Hepatol. 2016;28:369–382.
Naves JE, Llaó J, Ruiz-Cerulla A, et al. Long-term comparative efficacy of cyclosporine- or infliximab-based strategies for the management of steroid-refractory ulcerative colitis attacks. Inflamm Bowel Dis. 2014;20:1375–1381.
Szemes K, Soós A, Hegyi P, et al. Comparable long-term outcomes of cyclosporine and infliximab in patients with steroid-refractory acute severe ulcerative colitis: a meta-analysis. Front Med (Lausanne). 2019;6:338.
Golovics PA, Lakatos L, Mandel MD, et al. Does hospitalization predict the disease course in ulcerative colitis? Prevalence and predictors of hospitalization and re-hospitalization in ulcerative colitis in a population-based inception cohort (2000–2012). J Gastrointestin Liver Dis. 2015;24:287–292.
Ordás I, Domènech E, Mañosa M, et al. Post-operative morbidity and mortality of a cohort of steroid refractory acute severe ulcerative colitis: nationwide multicenter study of the GETECCU ENEIDA Registry. Am J Gastroenterol. 2018;113:1009–1016.
Ventham NT, Kennedy NA, Duffy A, et al. Comparison of mortality following hospitalisation for ulcerative colitis in Scotland between 1998–2000 and 2007–2009. Aliment Pharmacol Ther. 2014;39:1387–1397.
Opstelten JL, Vaartjes I, Bots ML, Oldenburg B. Mortality after first hospital admission for inflammatory bowel disease: a nationwide registry linkage study. Inflamm Bowel Dis. 2019;25:1692–1699.
Allison J, Herrinton LJ, Liu L, Yu J, Lowder J. Natural history of severe ulcerative colitis in a community-based health plan. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc. 2008;6:999–1003.
Briggs AH, Goeree R, Blackhouse G, O’Brien BJ. Probabilistic analysis of cost-effectiveness models: choosing between treatment strategies for gastroesophageal reflux disease. Med Decis Mak. 2002;22:290–308.
Ollendorf DA, Lidsky L. Infliximab drug and infusion costs among patients with Crohn’s disease in a commercially-insured setting. Am J Ther. 2006;13:502–506.
Nguyen NH, Koola J, Dulai PS, Prokop LJ, Sandborn WJ, Singh S. Rate of risk factors for and interventions to reduce hospital readmission in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol: Off Clin Pract J Am Gastroenterol Assoc.. 2019;18:1939–1948.
Acknowledgments
Parambir S. Dulai is supported by an American Gastroenterology Association Research Scholar Award. This study was supported in part by NIDDK-funded San Diego Digestive Diseases Research Center (P30 DK120515).
Author information
Authors and Affiliations
Contributions
PSD and VJ created the study concept and design, PSD acquired the data from literature for estimates, PSD conducted the model building and analyses, PSD and VJ drafted and finalized the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
PSD reports consulting and research grants from Takeda, Janssen, Pfizer, Abbvie, Buhlmann, Polymedco. VJ reports consulting fees from Abbvie, Eli Lilly, GlaxoSmithKline, Arena pharmaceuticals, Genetech, Pendopharm, Sandoz, Merck, Takeda, Janssen, Robarts Clinical Trials, Topivert, Celltrion; speakers reports fees from Takeda, Janssen, Shire, Ferring, Abbvie, Pfizer.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure
1: Two-way sensitivity analysis for rates of endoscopic improvement with hyperbaric oxygen therapy and infliximab when comparing hyperbaric oxygen therapy in combination with intravenous steroids versus up-front infliximab on day 1 plus steroids of hospitalization. HBOT: Hyperbaric oxygen therapy; IFX: infliximab; pHBOTMH: probability of achieving endoscopic improvements (Mayo endoscopic sub-score 0 or 1) with HBOT; pIFXMH: probability of achieving endoscopic improvements with infliximab; WTP: willingness-to-pay (TIFF 3804 kb)
Rights and permissions
About this article
Cite this article
Dulai, P.S., Jairath, V. A Microsimulation Model to Project the 5-Year Impact of Using Hyperbaric Oxygen Therapy for Ulcerative Colitis Patients Hospitalized for Acute Flares. Dig Dis Sci 66, 3740–3752 (2021). https://doi.org/10.1007/s10620-020-06707-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06707-3