Abstract
Background and Aims
DNA damage-regulated autophagy modulator 1 (DRAM1) is required for induction of autophagy and apoptosis. However, the influence of DRAM1 on the pathogenesis of inflammatory bowel disease (IBD) has not been explored.
Methods
DRAM1 expression was examined in the intestinal mucosa of patients with IBD and colons of colitis mice. We used a recombinant adeno-associated virus carrying small hairpain DRAM1 to knock down the DRAM1 gene to treat colitis in the mice. The effect of DRAM1 on autophagy and apoptosis of intestinal epithelial cells was explored. DRAM1-mediated interaction with the c-Jun N-terminal kinase (JNK) pathway was also examined.
Results
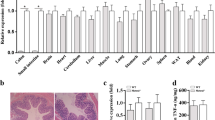
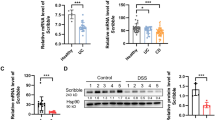
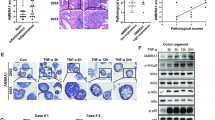
DRAM1 expression in the intestinal mucosa of the IBD patients was higher than that in the control participates. DRAM1 expression in the inflammatory cells in patients with Crohn’s disease (CD) was lower than that in patients with ulcerative colitis (UC). Additionally, DRAM1 expression was correlated with the Simple Endoscopic Score for CD and the Mayo endoscopic score for UC. Serum levels of DRAM1 in the IBD group were substantially higher than those in the normal group. The knockdown of DRAM1 could alleviate colitis symptoms in mice. In in vitro experiments, knocking down DRAM1 could reduce autophagy and apoptosis levels. Mechanistically, DRAM1 may participate in the regulation of these two processes by positively regulating JNK activation.
Conclusions
During intestinal inflammation, the upregulation of DRAM1 may promote the activation of JNK and further aggravate intestinal epithelium damage.







Similar content being viewed by others
Abbreviations
- IBD:
-
Inflammatory bowel disease
- CD:
-
Crohn’s disease
- UC:
-
Ulcerative colitis
- DRAM1:
-
DNA damage-regulated autophagy modulator 1
- CRP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- SES-CD:
-
Simple endoscopic score for Crohn’s disease scoring system
- Mayo UC:
-
Mayo clinic endoscopic subscore system
- AAV-DRAM1-KD:
-
Adeno-associated virus (AAV)-mediated DRAM1 knockdown
- TNBS:
-
Trinitrobenzene sulfonic acid
- DSS:
-
Dextran sulfate sodium
- JNK:
-
c-Jun N-terminal kinase
References
Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–466.
Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434.
Buhner S, Buning C, Genschel J, et al. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC mutation? Gut.. 2006;55:342–347.
Cheru L, Saylor CF, Lo J. Gastrointestinal barrier breakdown and adipose tissue inflammation. Curr Obesity Rep. 2019;8:165–174.
Günther C, Neumann H, Neurath MF, Becker C. Apoptosis, necrosis and necroptosis: cell death regulation in the intestinal epithelium. Gut. 2013;62:1062–1071.
Pedersen J, LaCasse EC, Seidelin JB, Coskun M, Nielsen OH. Inhibitors of apoptosis (IAPs) regulate intestinal immunity and inflammatory bowel disease (IBD) inflammation. Trends Mol Med. 2014;20:652–665.
Li M, Zhang S, Qiu Y, et al. Upregulation of miR-665 promotes apoptosis and colitis in inflammatory bowel disease by repressing the endoplasmic reticulum stress components XBP1 and ORMDL3. Cell Death Dis. 2017;8:e2699.
Nenci A, Becker C, Wullaert A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature.. 2007;446:557–561.
Retnakumar SV, Muller S. Pharmacological autophagy regulators as therapeutic agents for inflammatory bowel diseases. Trends Mol Med. 2019;25:516–537.
Iida T, Onodera K, Nakase H. Role of autophagy in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2017;23:1944–1953.
Nys K, Agostinis P, Vermeire S. Autophagy: a new target or an old strategy for the treatment of Crohn’s disease? Nat Rev Gastroenterol Hepatol. 2013;10:395–401.
Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939.
Crighton D, Wilkinson S, Ryan KM. DRAM links autophagy to p53 and programmed cell death. Autophagy. 2007;3:72–74.
Wirtz S, Popp V, Kindermann M, et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc. 2017;12:1295–1309.
Zhang S, Zhong B, Chen M, et al. Epigenetic reprogramming reverses the malignant epigenotype of the MMP/TIMP axis genes in tumor cells. Int J Cancer. 2014;134:1583–1594.
Wang H, Chao K, Ng SC, et al. Pro-inflammatory miR-223 mediates the cross-talk between the IL23 pathway and the intestinal barrier in inflammatory bowel disease. Genome Biol. 2016;17:58.
Yu Q, Zhang S, Chao K, et al. E3 ubiquitin ligase RNF183 is a novel regulator in inflammatory bowel disease. J Crohns Colitis. 2016;10:713–725.
Polyak S, Mach A, Porvasnik S, et al. Identification of adeno-associated viral vectors suitable for intestinal gene delivery and modulation of experimental colitis. Am J Physiol Gastrointest Liver Physiol. 2012;302:G296–G308.
Shen HM, Pervaiz S. TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J. 2006;20:1589–1598.
Xu H, Yao Y, Su Z, et al. Endogenous HMGB1 contributes to ischemia-reperfusion-induced myocardial apoptosis by potentiating the effect of TNF-α/JNK. Am J Physiol Heart Circ Physiol. 2011;300:H913–H921.
Lorin S, Pierron G, Ryan KM, Codogno P, Djavaheri-Mergny M. Evidence for the interplay between JNK and p53-DRAM signalling pathways in the regulation of autophagy. Autophagy. 2010;6:153–154.
Goto Y, Kiyono H. Epithelial barrier: an interface for the cross-communication between gut flora and immune system. Immunol Rev. 2012;245:147–163.
Wells JM, Rossi O, Meijerink M, van Baarlen P. Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci USA. 2011;108:4607–4614.
Wang SL, Shao BZ, Zhao SB, et al. Impact of paneth cell autophagy on inflammatory bowel disease. Front Immunol. 2018;9:693.
Wu X, Qin Y, Zhu X, et al. Increased expression of DRAM1 confers myocardial protection against ischemia via restoring autophagy flux. J Mol Cell Cardiol. 2018;124:70–82.
Yu M, Jiang Y, Feng Q, Ouyang YA, Gan J. DRAM1 protects neuroblastoma cells from oxygen-glucose deprivation/reperfusion-induced injury via autophagy. Int J Mol Sci. 2014;15:19253–19264.
Galavotti S, Bartesaghi S, Faccenda D, et al. The autophagy-associated factors DRAM1 and p62 regulate cell migration and invasion in glioblastoma stem cells. Oncogene. 2013;32:699–712.
Yang W, Tang H, Zhang Y, et al. Meta-analysis followed by replication identifies loci in or near CDKN1B, TET3, CD80, DRAM1, and ARID5B as associated with systemic lupus erythematosus in Asians. Am J Hum Genetics. 2013;92:41–51.
Memmert S, Nogueira AVB, Damanaki A, et al. Damage-regulated autophagy modulator 1 in oral inflammation and infection. Clin Oral Investig. 2018;22:2933–2941.
Uo M, Hisamatsu T, Miyoshi J, et al. Mucosal CXCR4 + IgG plasma cells contribute to the pathogenesis of human ulcerative colitis through FcgammaR-mediated CD14 macrophage activation. Gut. 2013;62:1734–1744.
Kett K, Rognum TO, Brandtzaeg P. Mucosal subclass distribution of immunoglobulin G-producing cells is different in ulcerative colitis and Crohn’s disease of the colon. Gastroenterology. 1987;93:919–924.
Neurath MF. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol. 2019;20:970–979.
Jinno Y, Ohtani H, Nakamura S, et al. Infiltration of CD19+ plasma cells with frequent labeling of Ki-67 in corticosteroid-resistant active ulcerative colitis. Virchows Arch. 2006;448:412–421.
Crighton D, Wilkinson S, O’Prey J, et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–134.
Dirisina R, Katzman RB, Goretsky T, et al. p53 and PUMA independently regulate apoptosis of intestinal epithelial cells in patients and mice with colitis. Gastroenterology. 2011;141:1036–1045.
Funding
This study was funded by the National Natural Science Foundation of China (Grant Nos. 81630018, 82000519, and 81970483).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All applicable international and national guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the First Affiliated Hospital of Sun Yat-sen University ([2018]038).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Li, X., Li, Y. et al. DNA Damage-Regulated Autophagy Modulator 1 (DRAM1) Mediates Autophagy and Apoptosis of Intestinal Epithelial Cells in Inflammatory Bowel Disease. Dig Dis Sci 66, 3375–3390 (2021). https://doi.org/10.1007/s10620-020-06697-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06697-2