Abstract
Background
There is little known about stem cells in human non-neoplastic and neoplastic esophageal epithelia. We have demonstrated expression of linker threonine-phosphorylated Smad2/3 (pSmad2/3L-Thr), suggesting presence of stem-like cells in mouse esophageal epithelium, and identified presence of pSmad2/3L-Thr-positive cells that might function as cancer stem cells in mouse model of colorectal carcinoma.
Aims
We explore whether pSmad2/3L-Thr can be used as a biomarker for stem cells of human esophageal epithelia and/or neoplasms.
Methods
We have used esophageal tissues from inpatients undergoing endoscopic submucosal dissection and performed double immunofluorescent staining of pSmad2/3L-Thr and Ki67, CDK4, p63, Sox2, CK14, p53, ALDH1, CD44 or D2-40 after which the sections were stained with hematoxylin and eosin.
Results
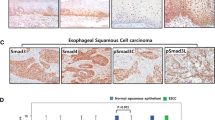
pSmad2/3L-Thr-positive cells showed immunohistochemical co-localization with CDK4, p63, CD44 and Sox2 in the basal and parabasal layers of non-neoplastic esophageal epithelia. In esophageal neoplasms, they showed immunohistochemical co-localization with p53, CDK4, ALDH1 and CD44. There was a significant increase in the percentage of pSmad2/3L-Thr-positive cells in the p53-positive neoplastic cell population with development of esophageal neoplasia. pSmad2/3L-Thr-positive cells localized to the lower section of low-grade intraepithelial neoplasia and were observed up to the upper section in carcinoma in situ. In invasive squamous cell carcinoma, they were scattered throughout the tumor with disappearance of polarity and were found in intraepithelial primary lesions and sites of submucosal and vessel invasion.
Conclusions
We determined significant expression of pSmad2/3L-Thr in human esophageal non-neoplastic and neoplastic epithelia, indicating that these are epithelial stem-like cells and cancer stem cells, respectively, that correlate with developing esophageal neoplasms.






Similar content being viewed by others
References
Bosman FT, International Agency for Research on Cancer. WHO classification of tumours of the digestive system. Lyon, France: International Agency for Research on Cancer; 2010.
Sarbia M, Porschen R, Borchard F, Horstmann O, Willers R, Gabbert HE. Incidence and prognostic significance of vascular and neural invasion in squamous cell carcinomas of the esophagus. Int J Cancer.. 1995;61:333–336.
Wang LD, Hong JY, Qiu SL, Gao H, Yang CS. Accumulation of p53 protein in human esophageal precancerous lesions: a possible early biomarker for carcinogenesis. Cancer Res.. 1993;53:1783–1787.
Volant A, Nousbaum JB, Giroux MA, et al. p53 protein accumulation in oesophageal squamous cell carcinomas and precancerous lesions. J Clin Pathol. 1995;48:531–534.
Kobayashi M, Kawachi H, Takizawa T, et al. p53 Mutation analysis of low-grade dysplasia and high-grade dysplasia/carcinoma in situ of the esophagus using laser capture microdissection. Oncology. 2006;71:237–245.
Suzuki H, Igarashi S, Nojima M, et al. IGFBP7 is a p53-responsive gene specifically silenced in colorectal cancer with CpG island methylator phenotype. Carcinogenesis. 2010;31:342–349.
Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344.
Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471.
Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791.
Wrana JL. Crossing Smads. Sci STKE. 2000;2000:re1.
Kretzschmar M, Doody J, Timokhina I, Massague J. A mechanism of repression of TGFbeta/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816.
Matsuura I, Denissova NG, Wang G, He D, Long J, Liu F. Cyclin-dependent kinases regulate the antiproliferative function of Smads. Nature.. 2004;430:226–231.
Mori S, Matsuzaki K, Yoshida K, et al. TGF-beta and HGF transmit the signals through JNK-dependent Smad2/3 phosphorylation at the linker regions. Oncogene. 2004;23:7416–7429.
Tarasewicz E, Jeruss JS. Phospho-specific Smad3 signaling: impact on breast oncogenesis. Cell Cycle. 2012;11:2443–2451.
Matsuzaki K. Smad3 phosphoisoform-mediated signaling during sporadic human colorectal carcinogenesis. Histol Histopathol. 2006;21:645–662.
Matsuzaki K, Kitano C, Murata M, et al. Smad2 and Smad3 phosphorylated at both linker and COOH-terminal regions transmit malignant TGF-beta signal in later stages of human colorectal cancer. Cancer Res. 2009;69:5321–5330.
Sapkota G, Knockaert M, Alarcon C, Montalvo E, Brivanlou AH, Massague J. Dephosphorylation of the linker regions of Smad1 and Smad2/3 by small C-terminal domain phosphatases has distinct outcomes for bone morphogenetic protein and transforming growth factor-beta pathways. J Biol Chem. 2006;281:40412–40419.
Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584.
Fukui T, Kishimoto M, Nakajima A, et al. The specific linker phosphorylation of Smad2/3 indicates epithelial stem cells in stomach; particularly increasing in mucosae of Helicobacter-associated gastritis. J Gastroenterol.. 2011;46:456–468.
Kishimoto M, Fukui T, Suzuki R, et al. Phosphorylation of smad2/3 at specific linker threonine indicates slow-cycling intestinal stem-like cells before reentry to cell cycle. Dig Dis Sci.. 2015;60:362–374.
Takahashi Y, Fukui T, Kishimoto M, et al. Phosphorylation of Smad2/3 at the specific linker threonine residue indicates slow-cycling esophageal stem-like cells before re-entry to the cell cycle. Dis Esophagus. 2016;29:107–115.
Suzuki R, Fukui T, Kishimoto M, et al. Smad2/3 linker phosphorylation is a possible marker of cancer stem cells and correlates with carcinogenesis in a mouse model of colitis-associated colorectal cancer. J Crohns Colitis. 2015;9:565–574.
Furukawa F, Matsuzaki K, Mori S, et al. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology. 2003;38:879–889.
Dunaway S, Rothaus A, Zhang Y, Luisa Kadekaro A, Andl T, Andl CD. Divide and conquer: two stem cell populations in squamous epithelia, reserves and the active duty forces. Int J Oral Sci. 2019;11:26.
Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol.. 2009;10:207–217.
Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell.. 2008;132:598–611.
Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer.. 1953;6:963–968.
Slaughter DP. Multicentric origin of intraoral carcinoma. Surgery.. 1946;20:133–146.
Hanson JA, Gillespie JW, Grover A, et al. Gene promoter methylation in prostate tumor-associated stromal cells. J Natl Cancer Inst.. 2006;98:255–261.
Roesch-Ely M, Nees M, Karsai S, et al. Proteomic analysis reveals successive aberrations in protein expression from healthy mucosa to invasive head and neck cancer. Oncogene. 2007;26:54–64.
Heaphy CM, Bisoffi M, Fordyce CA, et al. Telomere DNA content and allelic imbalance demonstrate field cancerization in histologically normal tissue adjacent to breast tumors. Int J Cancer.. 2006;119:108–116.
Lee YC, Wang HP, Wang CP, et al. Revisit of field cancerization in squamous cell carcinoma of upper aerodigestive tract: better risk assessment with epigenetic markers. Cancer Prev Res (Phila).. 2011;4:1982–1992.
Oka D, Yamashita S, Tomioka T, et al. The presence of aberrant DNA methylation in noncancerous esophageal mucosae in association with smoking history: a target for risk diagnosis and prevention of esophageal cancers. Cancer.. 2009;115:3412–3426.
Yakoub D, Keun HC, Goldin R, Hanna GB. Metabolic profiling detects field effects in nondysplastic tissue from esophageal cancer patients. Cancer Res.. 2010;70:9129–9136.
Roesch-Ely M, Leipold A, Nees M, et al. Proteomic analysis of field cancerization in pharynx and oesophagus: a prospective pilot study. J Pathol.. 2010;221:462–470.
Maehara R, Fujikura K, Takeuchi K, et al. SOX2-silenced squamous cell carcinoma: a highly malignant form of esophageal cancer with SOX2 promoter hypermethylation. Mod Pathol.. 2018;31:83–92.
Ishida H, Kasajima A, Kamei T, et al. SOX2 and Rb1 in esophageal small-cell carcinoma: their possible involvement in pathogenesis. Mod Pathol.. 2017;30:660–671.
Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–641.
Susaki E, Nakayama K, Nakayama KI. Cyclin D2 translocates p27 out of the nucleus and promotes its degradation at the G0-G1 transition. Mol Cell Biol. 2007;27:4626–4640.
Furukawa Y, Kikuchi J, Nakamura M, Iwase S, Yamada H, Matsuda M. Lineage-specific regulation of cell cycle control gene expression during haematopoietic cell differentiation. Br J Haematol.. 2000;110:663–673.
Daniely Y, Liao G, Dixon D, et al. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 2004;287:C171–181.
Zoller M. CD44, hyaluronan, the hematopoietic stem cell, and leukemia-initiating cells. Front Immunol. 2015;6:235.
Kalabis J, Oyama K, Okawa T, et al. A subpopulation of mouse esophageal basal cells has properties of stem cells with the capacity for self-renewal and lineage specification. J Clin Invest. 2008;118:3860–3869.
Weidner N, Moore DH 2nd, Vartanian R. Correlation of Ki-67 antigen expression with mitotic figure index and tumor grade in breast carcinomas using the novel “paraffin”-reactive MIB1 antibody. Hum Pathol. 1994;25:337–342.
Wrighton KH, Willis D, Long J, Liu F, Lin X, Feng XH. Small C-terminal domain phosphatases dephosphorylate the regulatory linker regions of Smad2 and Smad3 to enhance transforming growth factor-beta signaling. J Biol Chem. 2006;281:38365–38375.
Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567.
Zhao JS, Li WJ, Ge D, et al. Tumor initiating cells in esophageal squamous cell carcinomas express high levels of CD44. PLoS One. 2011;6:e21419.
Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–978.
Yang L, Ren Y, Yu X, et al. ALDH1A1 defines invasive cancer stem-like cells and predicts poor prognosis in patients with esophageal squamous cell carcinoma. Mod Pathol. 2014;27:775–783.
Acknowledgments
This study was supported by grant-in-aid for Research Grant (B) from Japanese Foundation for Research and Promotion of Endoscopy and grant-in-aid for Scientific Research (C) from Japan Society for the Promotion of Science (16K09330, 25460938).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no potential conflicts of interest exist.
Human and animal rights
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional (number 2012101) and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Horitani, S., Fukui, T., Tanimura, Y. et al. Specific Smad2/3 Linker Phosphorylation Indicates Esophageal Non-neoplastic and Neoplastic Stem-Like Cells and Neoplastic Development. Dig Dis Sci 66, 1862–1874 (2021). https://doi.org/10.1007/s10620-020-06489-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06489-8