Abstract
Background
Infected pancreatic necrosis is one of the most severe complications of acute pancreatitis (AP). The development of secondary infection doubles the risk of death during the late stage of necrotizing pancreatitis. Phagocytes play a major role in AP pathogenesis, as well as in local and systemic complications of the disease.
Aims
We aimed to investigate the relationship between quantitative and functional indices of circulating phagocyte at the time of admission and onset of infectious complications in patients with AP afterward.
Methods
A post hoc analysis of 97 patients with AP was conducted. The metabolic state of peripheral blood neutrophils and monocytes was analyzed based on their phagocytic activity and generation of reactive oxygen species (ROS), which were determined by flow cytometry on admission. The clinical end point was marked by onset of infectious complications of AP.
Results
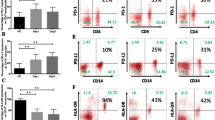
On admission, baseline values and reactivity reserve of monocyte and neutrophil phagocytic activity in AP patients, who developed septic complications, were substantially decreased, whereas monocyte ROS generation was dramatically increased as compared to the group without infectious processes. ROC curve was obtained both for neutrophil and monocyte phagocytosis reactivity reserve expressed as modulation coefficient values and categorized as the risk factor of infectious complications, showing an area under curve of 0.95 (P < 0.0001) and 0.84 (P < 0.0001), respectively.
Conclusions
Early (at the time of admission) detection of quantitative and functional indices of circulating phagocytes can be useful for the prediction of septic complications in SAP patients.


Similar content being viewed by others
References
Roberts SE, Morrison-Rees S, John A, Williams JG, Brown TH, Samuel DG. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology. 2017;17:155–165.
Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:175–184.
Maheshwari R, Subramanian RM. Severe acute pancreatitis and necrotizing pancreatitis. Crit Care Clin. 2016;32:279–290.
Boumitri C, Brown E, Kahaleh M. Necrotizing pancreatitis: current management and therapies. Clin Endosc. 2017;50:357–365.
Brown LA, Hore TA, Phillips AR, Windsor JA, Petrov MS. A systematic review of the extra-pancreatic infectious complications in acute pancreatitis. Pancreatology. 2014;14:436–443.
Werge M, Novovic S, Schmidt PN, Gluud LL. Infection increases mortality in necrotizing pancreatitis: a systematic review and meta-analysis. Pancreatology. 2016;16:698–707.
van Grinsven J, van Brunschot S, Bakker OJ, et al. Diagnostic strategy and timing of intervention in infected necrotizing pancreatitis: an international expert survey and case vignette study. HPB (Oxford). 2016;18:49–56.
Popa CC. Prognostic biological factors in severe acute pancreatitis. J Med Life. 2014;7:525–528.
Staubli SM, Oertli D, Nebiker CA. Laboratory markers predicting severity of acute pancreatitis. Clin Res Hepatol Gastroenterol. 2018;52:273–283.
Wilson J, Zarabi S. BET 1: SIRS criteria as a way of predicting mortality in acute pancreatitis. Emerg Med J. 2017;34:621–622.
Yang N, Li B, Ye B, et al. The long-term quality of life in patients with persistent inflammation-immunosuppression and catabolism syndrome after severe acute pancreatitis: a retrospective cohort study. J Crit Care. 2017;42:101–106.
Kylänpää ML, Repo H, Puolakkainen PA. Inflammation and immunosuppression in severe acute pancreatitis. World J Gastroenterol. 2010;16:2867–2872.
Yang ZW, Meng XX, Xu P. Central role of neutrophil in the pathogenesis of severe acute pancreatitis. J Cell Mol Med. 2015;19:2513–2520.
Gukovskaya AS, Gukovsky I, Algül H, Habtezion A. Autophagy, inflammation, and immune dysfunction in the pathogenesis of pancreatitis. Gastroenterology. 2017;153:1212–1226.
Liu G, Tao J, Zhu Z, Wang W. The early prognostic value of inflammatory markers in patients with acute pancreatitis. Clin Res Hepatol Gastroenterol. 2018;43:330–337.
Yu ZX, Chen XC, Zhang BY, Liu N, Gu Q. Association between HLA-DR expression and multidrug-resistant infection in patients with severe acute pancreatitis. Curr Med Sci. 2018;38:449–454.
Zhang H, Ling XL, Wu YY, et al. CD64 expression is increased in patients with severe acute pancreatitis: clinical significance. Gut Liver. 2014;8:445–451.
Lin J, Li Z, Zheng Y, et al. Elevated presepsin levels are associated with severity and prognosis of severe acute pancreatitis. Clin Lab. 2016;62:1699–1708.
Chakraborty M, Hickey AJ, Petrov MS, et al. Mitochondrial dysfunction in peripheral blood mononuclear cells in early experimental and clinical acute pancreatitis. Pancreatology. 2016;16:739–747.
Vinnik YS, Dunaevskaya SS, Antufrieva DA. Diagnostic value of the integral hematological indexes and chemiluminescence of neutrophils in severe acute pancreatitis. Eksp Klin Gastroenterol. 2016;10:86–90.
El-Benna J, Hurtado-Nedelec M, Marzaioli V, Marie JC, Gougerot-Pocidalo MA, Dang PM. Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev. 2016;273:180–193.
Leliefeld PH, Wessels CM, Leenen LP, Koenderman L, Pillay J. The role of neutrophils in immune dysfunction during severe inflammation. Crit Care. 2016;20:73.
Kuuliala K, Penttilä AK, Kaukonen KM, et al. Signalling profiles of blood leucocytes in sepsis and in acute pancreatitis in relation to disease severity. Scand J Immunol. 2018;87:88–98.
Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111.
Talukdar R, Clemens M, Vege SS. Moderately severe acute pancreatitis: prospective validation of this new subgroup of acute pancreatitis. Pancreas. 2012;41:306–309.
Rudyk M, Fedorchuk O, Susak Y, Nowicky Y, Skivka L. Introduction of antineoplastic drug NSC631570 in an inpatient and outpatient setting: comparative evaluation of biological effects. Asian J Pharm Sci. 2016;11:308–317.
Honda T, Uehara T, Matsumoto G, Arai S, Sugano M. Neutrophil left shift and white blood cell count as markers of bacterial infection. Clin Chim Acta. 2016;457:46–53.
Garcia LG, Lemaire S, Kahl BC, et al. Influence of the protein kinase C activator phorbol myristate acetate on the intracellular activity of antibiotics against hemin- and menadione-auxotrophic small-colony variant mutants of Staphylococcus aureus and their wild-type parental strain in human THP-1 cells. Antimicrob Agents Chemother. 2012;56:6166–6174.
Larsen EC, DiGennaro JA, Saito N, et al. Differential requirement for classic and novel PKC isoforms in respiratory burst and phagocytosis in RAW 264.7 cells. J Immunol. 2000;165:2809–2817.
Schepers NJ, Bakker OJ, Besselink MG, et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut. 2019;68:1044–1051.
Wang M, Wei A, Guo Q, et al. Clinical outcomes of combined necrotizing pancreatitis versus extrapancreatic necrosis alone. Pancreatology. 2016;16:57–65.
Janeway CA, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th ed. New York: Garland Science; 2001.
Watanabe T, Kudo M, Strober W. Immunopathogenesis of pancreatitis. Mucosal Immunol. 2017;10:283–298.
Bartel M, Hänsch GM, Giese T, et al. Abnormal crosstalk between pancreatic acini and macrophages during the clearance of apoptotic cells in chronic pancreatitis. J Pathol. 2008;215:195–203.
Landahl P, Ansari D, Andersson R. Severe acute pancreatitis: gut barrier failure, systemic inflammatory response, acute lung injury, and the role of the mesenteric lymph. Surg Infect (Larchmt). 2015;16:651–656.
Cardinale F, Chinellato I, Caimmi S, et al. Perioperative period: immunological modifications. Int J Immunopathol Pharmacol. 2011;24:S3–S12.
Zhang ML, Jiang YF, Wang XR, et al. Different phenotypes of monocytes in patients with new-onset mild acute pancreatitis. World J Gastroenterol. 2017;23:1477–1488.
Zhu F, Yue W, Wang Y. The nuclear factor kappa B (NF-κB) activation is required for phagocytosis of Staphylococcus aureus by RAW 264.7 cells. Exp Cell Res. 2014;327:256–263.
Oiva J, Mustonen H, Kylänpää ML, et al. Patients with acute pancreatitis complicated by organ failure show highly aberrant monocyte signaling profiles assessed by phospho-specific flow cytometry. Crit Care Med. 2010;38:1702–1708.
Oiva J, Mustonen H, Kylänpää ML, et al. Patients with acute pancreatitis complicated by organ dysfunction show abnormal peripheral blood polymorphonuclear leukocyte signaling. Pancreatology. 2013;13:118–124.
Masamune A, Kume K, Kikuta K, et al. 651C/T promoter polymorphism in the CD14 gene is associated with severity of acute pancreatitis in Japan. J Gastroenterol. 2010;45:225–233.
Matas-Cobos AM, Redondo-Cerezo E, Alegría-Motte C, et al. The role of Toll-like receptor polymorphisms in acute pancreatitis occurrence and severity. Pancreas. 2015;44:429–433.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Susak, Y.M., Dirda, O.O., Fedorchuk, O.G. et al. Infectious Complications of Acute Pancreatitis Is Associated with Peripheral Blood Phagocyte Functional Exhaustion. Dig Dis Sci 66, 121–130 (2021). https://doi.org/10.1007/s10620-020-06172-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06172-y