Abstract
Background
The incidence of Barrett’s esophagus (BE) and esophageal adenocarcinoma (EAC) continues to rise, and risk stratification of patients with BE is needed. Impaired esophageal motility is associated with gastroesophageal reflux disease; however, whether esophageal dysmotility is a risk factor for dysplasia progression in BE is incompletely understood. This study aimed to characterize esophageal motility patterns in patients with BE and identify physiologic factors associated with dysplasia progression in BE.
Methods
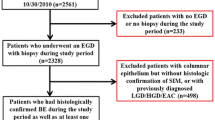
This multicenter retrospective study assessed data from adult patients with histologically confirmed BE who underwent high-resolution esophageal manometry from 1/2014 to 1/2018 at four tertiary care centers. Longitudinal data were collected when available among patients with non-dysplastic BE (NDBE) and separated as: no dysplastic progression or positive dysplastic progression. Multivariable logistic regression assessed for independent predictors of dysplasia progression.
Results
Among 193 patients, histology at index endoscopy identified 152 (79%) NDBE, 23 (12%) low-grade dysplasia, 14 (7%) high-grade dysplasia, and 4 (2%) EAC. Ninety-eight (51%) had abnormal esophageal motor function on manometry. Longitudinal data were available for 84 of 152 patients with initial NDBE. Twelve (14%) exhibited dysplastic progression to low-grade (6) or high-grade (6) dysplasia. Mean esophageal distal contractile integral was lower for patients that progressed [455 mmHg s cm (SD 515)] compared with patients who did not progress [987 mmHg s cm (SD 953); aOR 1.21 (95% CI 1.01, 1.44)].
Conclusion
In this retrospective study of 193 BE patients, the majority exhibited abnormal esophageal motor function. Reduced esophageal contractility was independently associated with dysplastic progression in BE. Characterizing esophageal physiology in BE may help to risk stratify patients.

Similar content being viewed by others
Abbreviations
- BE:
-
Barrett’s esophagus
- EAC:
-
Esophageal adenocarcinoma
- NDBE:
-
Non-dysplastic Barrett’s esophagus
- LGD:
-
Low-grade dysplasia
- HGD:
-
High-grade dysplasia
- PPI:
-
Proton pump inhibitor
- GERD:
-
Gastroesophageal reflux disease
- BMI:
-
Body mass index
- IRP:
-
Integrated relaxation pressure
- DCI:
-
Distal contractile integral
- EGJ-CI:
-
Esophagogastric junction contractile integral
- PSPWi:
-
Post-reflux swallow peristaltic wave index
- aOR:
-
Adjusted odds ratio
- CI:
-
Confidence interval
- SD:
-
Standard deviation
References
Hameeteman W, Tytgat GN, Houthoff HJ, van den Tweel JG. Barrett’s esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249–1256.
Kestens C, Offerhaus GJ, van Baal JW, Siersema PD. Patients with Barrett’s esophagus and persistent low-grade dysplasia have an increased risk for high-grade dysplasia and cancer. Clin Gastroenterol Hepatol. 2016;14:956.e1–962.e1.
Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol. 2012;23:3155–3162.
Desai M, Lieberman DA, Kennedy KF, et al. Increasing prevalence of high-grade dysplasia and adenocarcinoma on index endoscopy in Barrett’s esophagus over the past 2 decades: data from a multicenter U.S. consortium. Gastrointest Endosc. 2018;89:257–263.
Codipilly DC, Chandar AK, Singh S, et al. The effect of endoscopic surveillance in patients with Barrett’s esophagus: a systematic review and meta-analysis. Gastroenterology. 2018;154:2068e5–2086e5.
Krishnamoorthi R, Singh S, Ragunathan K, et al. Factors associated with progression of Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16:1046e8–1055e8.
Thrift AP, Garcia JM, El-Serag HB. A multibiomarker risk score helps predict risk for Barrett’s esophagus. Clin Gastroenterol Hepatol. 2014;12:1267–1271.
Rubenstein JH, Morgenstern H, Appelman H, et al. Prediction of Barrett’s esophagus among men. Am J Gastroenterol. 2013;108:353–362.
Oberg S, DeMeester TR, Peters JH, et al. The extent of Barrett’s esophagus depends on the status of the lower esophageal sphincter and the degree of esophageal acid exposure. J Thorac Cardiovasc Surg. 1999;117:572–580.
Helman L, Biccas BN, Lemme EM, Novais P, Fittipaldi V. Esophageal manometry findings and degree of acid exposure in short and long Barrett’s esophagus. Arq Gastroenterol. 2012;49:64–68.
Blevins CH, Iyer PG, Vela MF, Katzka DA. The esophageal epithelial barrier in health and disease. Clin Gastroenterol Hepatol. 2018;16:608–617.
Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–174.
Wani S, Gaddam S. Editorial: Best practices in surveillance of Barrett’s esophagus. Am J Gastroenterol. 2017;112:1056–1060.
Parasa S, Vennalaganti S, Gaddam S, et al. Development and validation of a model to determine risk of progression of Barrett’s esophagus to neoplasia. Gastroenterology. 2018;154:1282e2–1289e2.
Sanagapalli S, Emmanuel A, Leong R, et al. Impaired motility in Barrett’s esophagus: a study using high-resolution manometry with physiologic challenge. Neurogastroenterol Motil. 2018. https://doi.org/10.1111/nmo.13330.
Bazin C, Benezech A, Alessandrini M, Grimaud JC, Vitton V. Esophageal motor disorders are a strong and independent associated factor of Barrett’s esophagus. J Neurogastroenterol Motil. 2018;24:216–225.
Farre R, van Malenstein H, De Vos R, et al. Short exposure of oesophageal mucosa to bile acids, both in acidic and weakly acidic conditions, can impair mucosal integrity and provoke dilated intercellular spaces. Gut. 2008;57:1366–1374.
Xiao Y, Kahrilas PJ, Kwasny MJ, et al. High-resolution manometry correlates of ineffective esophageal motility. Am J Gastroenterol. 2012;107:1647–1654.
Kahrilas PJ. Esophageal motor activity and acid clearance. Gastroenterol Clin North Am. 1990;19:537–550.
Frazzoni M, de Bortoli N, Frazzoni L, et al. The added diagnostic value of postreflux swallow-induced peristaltic wave index and nocturnal baseline impedance in refractory reflux disease studied with on-therapy impedance-pH monitoring. Neurogastroenterol Motil. 2017;29.
Patel A, Wang D, Sainani N, Sayuk GS, Gyawali CP. Distal mean nocturnal baseline impedance on pH-impedance monitoring predicts reflux burden and symptomatic outcome in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2016;44:890–898.
Wang D, Patel A, Mello M, Shriver A, Gyawali CP. Esophagogastric junction contractile integral (EGJ-CI) quantifies changes in EGJ barrier function with surgical intervention. Neurogastroenterol Motil. 2016;28:639–646.
Funding
None related to this manuscript.
Author information
Authors and Affiliations
Contributions
RY, SW, JT, FQ, SE, SB, JP, SK, PG, PMK contributed to study concept and design; RY and SW involved in study oversight; RY, SW, ZV, JT, FQ, SE, SB, JP, SK, PG, NJS, PMK participated in acquisition of data; RY, SW, AK took part in analysis and interpretation of data; RY, SW, AK, JT, FQ, SE, SB, JP, SK, PG, PMK involved in drafting of manuscript; RY, SW, AK, JT, FQ, SE, SB, JP, SK, PG, NJS, PMK took part in critical revision of the manuscript for important intellectual content; RY, SW, AK, JT, FQ, SE, SB, JP, SK, PG, NJS, PMK participated in finalization of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
JEP: Consultant for Crospon, Ironwood, Torax, Astra Zeneca, Takeda, Impleo, Medtronic, Sandhill.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yadlapati, R., Triggs, J., Quader, F. et al. Reduced Esophageal Contractility Is Associated with Dysplasia Progression in Barrett’s Esophagus: A Multicenter Cohort Study. Dig Dis Sci 65, 3631–3638 (2020). https://doi.org/10.1007/s10620-020-06098-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06098-5