Abstract
Background
Teduglutide, a glucagon-like peptide 2 analog, has demonstrated efficacy in treating adult patients with short bowel syndrome (SBS) and dependence on parenteral nutrition (PN), but its role in chronic malabsorptive states that do not necessitate PN remains uncertain.
Aims
To evaluate teduglutide use beyond its approved indications and to discuss the results of this adjunctive treatment in patients resistant to established therapy.
Results
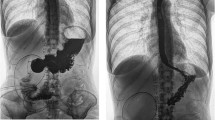
This series reports four patients treated with teduglutide off-label. The first case had Crohn’s disease (CD) with persistent colocutaneous fistulae that demonstrated complete closure after 8 months of teduglutide therapy. The second case involved a PN-dependent CD patient with persistent fistulae and intra-abdominal abscesses who weaned off PN and had a significant improvement in her nutritional status after 3 months of teduglutide therapy. The third case had CD complicated by severe malnutrition and previous PN-associated line infections, but by 9 months of teduglutide therapy, she gained 5 kg and no longer required re-initiation of PN. The fourth case had a high-output diverting ileostomy with resultant impaired healing of a stage IV decubitus ulcer, and after 2 months of therapy, the patient’s pre-albumin increased by 250% and the ulcer had decreased by 40% in size.
Conclusion
The use of teduglutide might be broadened to include patients with functional SBS not meeting strict criteria for intestinal failure. Further studies should evaluate the efficacy of teduglutide in patients who may require short-term small intestine rehabilitation or who have chronically impaired absorptive capacity not yet requiring PN.
Similar content being viewed by others
References
Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA. 1996;93:7911–7916.
Brubaker PL, Izzo A, Hill M, Drucker DJ. Intestinal function in mice with small bowel growth induced by glucagon-like peptide-2. Am J Physiol. 1997;272:E1050–E1058.
Jeppesen PB, Hartmann B, Thulesen J, et al. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology. 2001;120:806–815.
Dube PE, Forse CL, Bahrami J, Brubaker PL. The essential role of insulin-like growth factor-1 in the intestinal tropic effects of glucagon-like peptide-2 in mice. Gastroenterology. 2006;131:589–605.
Yusta B, Holland D, Koehler JA, et al. ErbB signaling is required for the proliferative actions of GLP-2 in the murine gut. Gastroenterology. 2009;137:986–996.
Bremholm L, Hornum M, Henriksen BM, Larsen S, Holst JJ. Glucagon-like peptide-2 increases mesenteric blood flow in humans. Scand J Gastroenterol. 2009;44:314–319.
Sigalet DL, Wallace LE, Holst JJ, et al. Enteric neural pathways mediate the anti-inflammatory actions of glucagon-like peptide 2. Am J Physiol Gastrointest Liver Physiol. 2007;293:G211–G221.
Perez A, Duxbury M, Rocha FG, et al. Glucagon-like peptide 2 is an endogenous mediator of postresection intestinal adaptation. JPEN J Parenter Enter Nutr. 2005;29:97–101.
Carroll R, Benedetti E, Schowalter J, Buchman A. Management and complications of short bowel syndrome: an updated review. Curr Gastroenterol Rep. 2016;18:1–13.
Jeppesen PB, Pertkiewicz M, Messing B, et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143:1481.e3.
Seidner DL, Fujioka K, Boullata JI, Iyer K, Lee HM, Ziegler TR. Reduction of parenteral nutrition and hydration support and safety with long-term teduglutide treatment in patients with short bowel syndrome-associated intestinal failure: STEPS-3 study. Nutr Clin Pract Off Publ Am Soc Parent Enter Nutr. 2018;33:520–527.
Schwartz LK, O’Keefe SJ, Fujioka K, et al. Long-term teduglutide for the treatment of patients with intestinal failure associated with short bowel syndrome. Clin Transl Gastroenterol. 2016;7:e142.
Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O’Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60:902–914.
O’Keefe SJ, Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B. Safety and efficacy of teduglutide after 52 weeks of treatment in patients with short bowel intestinal failure. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2013;11:815-23.e1-3.
Buchman AL, Katz S, Fang JC, Bernstein CN, Abou-Assi SG. Teduglutide, a novel mucosally active analog of glucagon-like peptide-2 (GLP-2) for the treatment of moderate to severe Crohn’s disease. Inflamm Bowel Dis. 2010;16:962–973.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All authors have contributed substantially to this report and have approved its final version. No authors disclose a conflict of interest with regard to what is presented in this series.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
George, A.T., Li, B.H. & Carroll, R.E. Off-Label Teduglutide Therapy in Non-intestinal Failure Patients with Chronic Malabsorption. Dig Dis Sci 64, 1599–1603 (2019). https://doi.org/10.1007/s10620-019-5473-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-019-5473-5